10.E: Acid-Base Equilibria (Exercises)
- Last updated
- Save as PDF
- Page ID
- 451648
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)14.1: Brønsted-Lowry Acids and Bases
Q14.1.1
Write equations that show NH3 as both a conjugate acid and a conjugate base.
S14.1.1
One example for NH3 as a conjugate acid: \(\ce{NH2- + H+ ⟶ NH3}\); as a conjugate base: \(\ce{NH4+}(aq)+\ce{OH-}(aq)⟶\ce{NH3}(aq)+\ce{H2O}(l)\)
Q14.1.2
Write equations that show \(\ce{H2PO4-}\) acting both as an acid and as a base.
Q14.1.3
Show by suitable net ionic equations that each of the following species can act as a Brønsted-Lowry acid:
- \(\ce{H3O+}\)
- HCl
- NH3
- CH3CO2H
- \(\ce{NH4+}\)
- \(\ce{HSO4-}\)
S14.1.3
- \(\ce{H3O+}(aq)⟶\ce{H+}(aq)+ \ce{H_2O}_{(l)}\) ;
- \(\ce{HCl}(l)⟶\ce{H+}(aq)+\ce{Cl-}(aq)\);
- \(\ce{NH3}(aq)⟶\ce{H+}(aq)+\ce{NH2-}(aq)\);
- \(\ce{CH3CO2H}(aq)⟶\ce{H+}(aq)+\ce{CH3CO2-}(aq)\);
- \(\ce{NH4+}(aq)⟶\ce{H+}(aq)+\ce{NH3}(aq)\);
- \(\ce{HSO4-}(aq)⟶\ce{H+}(aq)+\ce{SO4^2-}(aq)\)
Q14.1.4
Show by suitable net ionic equations that each of the following species can act as a Brønsted-Lowry acid:
- HNO3
- \(\ce{PH4+}\)
- H2S
- CH3CH2COOH
- \(\ce{H2PO4-}\)
- HS−
Q14.1.5
Show by suitable net ionic equations that each of the following species can act as a Brønsted-Lowry base:
- H2O
- \(\ce{OH-}\)
- NH3
- CN−
- S2−
- \(\ce{H2PO4-}\)
S14.1.5
- \(\ce{H_2O}_{(l)} + \ce{H^+} (aq)⟶\ce{H3O+}(aq)\)
- \(\ce{OH-} (aq) + \ce{H^+} (aq)⟶ \ce{H_2O}_{(l)}\)
- \(\ce{NH3}(aq) + \ce{H^+} (aq)⟶\ce{NH4+}(aq)\);
- \(\ce{CN-}(aq) + \ce{H^+} (aq)⟶\ce{HCN}(aq)\)
- \(\ce{S^2-}(aq) + \ce{H^+} (aq)⟶\ce{HS-}(aq)\)
- \(\ce{H2PO4-}(aq) + \ce{H^+} (aq)⟶\ce{H3PO4}(aq)\)
Q14.1.6
Show by suitable net ionic equations that each of the following species can act as a Brønsted-Lowry base:
- HS−
- \(\ce{PO4^3-}\)
- \(\ce{NH2-}\)
- C2H5OH
- O2−
- \(\ce{H2PO4-}\)
Q14.1.7
What is the conjugate acid of each of the following? What is the conjugate base of each?
- \(\ce{OH-}\)
- H2O
- \(\ce{HCO3-}\)
- NH3
- \(\ce{HSO4-}\)
- H2O2
- HS−
- \(\ce{H5N2+}\)
S14.1.7
H2O, O2−; H3O+, \(\ce{OH^-}\) ; H2CO3, \(\ce{CO3^2-}\); \(\ce{NH4+}\), \(\ce{NH2-}\); H2SO4, \(\ce{SO4^2-}\); \(\ce{H3O2+}\), \(\ce{HO2-}\); H2S; S2−; \(\ce{H6N2^2+}\), H4N2
Q14.1.8
What is the conjugate acid of each of the following? What is the conjugate base of each?
- H2S
- \(\ce{H2PO4-}\)
- PH3
- HS−
- \(\ce{HSO3-}\)
- \(\ce{H3O2+}\)
- H4N2
- CH3OH
Q14.1.9
Identify and label the Brønsted-Lowry acid, its conjugate base, the Brønsted-Lowry base, and its conjugate acid in each of the following equations:
- \(\ce{HNO3 + H2O ⟶ H3O+ + NO3-}\)
- \(\ce{CN- + H2O ⟶ HCN + OH-}\)
- \(\ce{H2SO4 + Cl- ⟶ HCl + HSO4-}\)
- \(\ce{HSO4- + OH- ⟶ SO4^2- + H2O}\)
- \(\ce{O^2- + H2O ⟶ 2OH-}\)
- \(\ce{[Cu(H2O)3(OH)]+ + [Al(H2O)6]^3+ ⟶ [Cu(H2O)4]^2+ + [Al(H2O)5(OH)]^2+}\)
- \(\ce{H2S + NH2- ⟶ HS- + NH3}\)
S14.1.9
The labels are Brønsted-Lowry acid = BA; its conjugate base = CB; Brønsted-Lowry base = BB; its conjugate acid = CA. HNO3(BA), H2O(BB), H3O+(CA), \(\ce{NO3- (CB)}\); CN−(BB), H2O(BA), HCN(CA), \(\ce{OH^-}\) (CB); H2SO4(BA), Cl−(BB), HCl(CA), \(\ce{HSO4- (CB)}\); \(\ce{HSO4- (BA)}\), OH-(BB), \(\ce{SO4^2- (CB)}\), H2O(CA); O2−(BB), H2O(BA) \(\ce{OH^-}\) (CB and CA); [Cu(H2O)3(OH)]+(BB), [Al(H2O)6]3+(BA), [Cu(H2O)4]2+(CA), [Al(H2O)5(OH)]2+(CB); H2S(BA), \(\ce{NH2- (BB)}\), HS−(CB), NH3(CA)
Q14.1.10
Identify and label the Brønsted-Lowry acid, its conjugate base, the Brønsted-Lowry base, and its conjugate acid in each of the following equations:
- \(\ce{NO2- + H2O ⟶ HNO2 + OH-}\)
- \(\ce{HBr + H2O ⟶ H3O+ + Br-}\)
- \(\ce{HS- + H2O ⟶ H2S + OH-}\)
- \(\ce{H2PO4- + OH- ⟶HPO4^2- + H2O}\)
- \(\ce{H2PO4- + HCl ⟶ H3PO4 + Cl-}\)
- \(\ce{[Fe(H2O)5(OH)]^2+ + [Al(H2O)6]^3+ ⟶ [Fe(H2O)6]^3+ + [Al(H2O)5(OH)]^2+}\)
- \(\ce{CH3OH + H- ⟶ CH3O- + H2}\)
Q14.1.11
What are amphiprotic species? Illustrate with suitable equations.
S14.1.11
Amphiprotic species may either gain or lose a proton in a chemical reaction, thus acting as a base or an acid. An example is H2O.
- As an acid: \(\ce{H2O}(aq) + \ce{NH3}(aq) \rightleftharpoons \ce{NH4+}(aq) + \ce{OH-}(aq)\).
- As a base: \(\ce{H2O}(aq) + \ce{HCl}(aq) \rightleftharpoons \ce{H3O+}(aq) + \ce{Cl-}(aq)\)
Q14.1.12
State which of the following species are amphiprotic and write chemical equations illustrating the amphiprotic character of these species:
- H2O
- \(\ce{H2PO4-}\)
- S2−
- \(\ce{CO3^2-}\)
- \(\ce{HSO4-}\)
Q14.1.13
State which of the following species are amphiprotic and write chemical equations illustrating the amphiprotic character of these species.
- NH3
- \(\ce{HPO4-}\)
- Br−
- \(\ce{NH4+}\)
- \(\ce{ASO4^3-}\)
S14.113
amphiprotic: \(\ce{NH3 + H3O+ ⟶ NH4OH + H2O}\), \(\ce{NH3 + OCH3- ⟶ NH2- + CH3OH}\); \(\ce{HPO4^2- + OH- ⟶ PO4^3- + H2O}\), \(\ce{HPO4^2- + HClO4 ⟶ H2PO4- + ClO4-}\); not amphiprotic: Br−; \(\ce{NH4+}\); \(\ce{AsO4^3-}\)
Q14.1.14
Is the self ionization of water endothermic or exothermic? The ionization constant for water (Kw) is \(2.9 \times 10^{-14}\) at 40 °C and \(9.6 \times 10^{-14}\) at 60 °C.
14.2: pH and pOH
Q14.2.1
Explain why a sample of pure water at 40 °C is neutral even though [H3O+] = 1.7 × 10−7 M. Kw is 2.9 × 10−14 at 40 °C.
S14.2.1
In a neutral solution [H3O+] = [OH−]. At 40 °C,
[H3O+] = [OH−] = (2.910−14)1/2 = 1.7 × 10−7.
Q14.2.2
The ionization constant for water (Kw) is 2.9 × 10−14 at 40 °C. Calculate [H3O+], [OH−], pH, and pOH for pure water at 40 °C.
Q14.2.3
The ionization constant for water (Kw) is 9.614 × 10−14 at 60 °C. Calculate [H3O+], [OH−], pH, and pOH for pure water at 60 °C.
S14.2.3
x = 3.101 × 10−7 M = [H3O+] = [OH−]
pH = -log 3.101 × 10−7 = −(−6.5085) = 6.5085
pOH = pH = 6.5085
Q14.2.4
Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely:
- 0.200 M HCl
- 0.0143 M NaOH
- 3.0 M HNO3
- 0.0031 M Ca(OH)2
Q14.2.5
Calculate the pH and the pOH of each of the following solutions at 25 °C for which the substances ionize completely:
- 0.000259 M HClO4
- 0.21 M NaOH
- 0.000071 M Ba(OH)2
- 2.5 M KOH
S14.2.5
pH = 3.587; pOH = 10.413; pH = 0.68; pOH = 13.32; pOH = 3.85; pH = 10.15; pH = −0.40; pOH = 14.4
Q14.2.6
What are the pH and pOH of a solution of 2.0 M HCl, which ionizes completely?
Q14.2.6
What are the hydronium and hydroxide ion concentrations in a solution whose pH is 6.52?
S14.2.6
[H3O+] = 3.0 × 10−7 M; [OH−] = 3.3 × 10−8 M
Q14.2.7
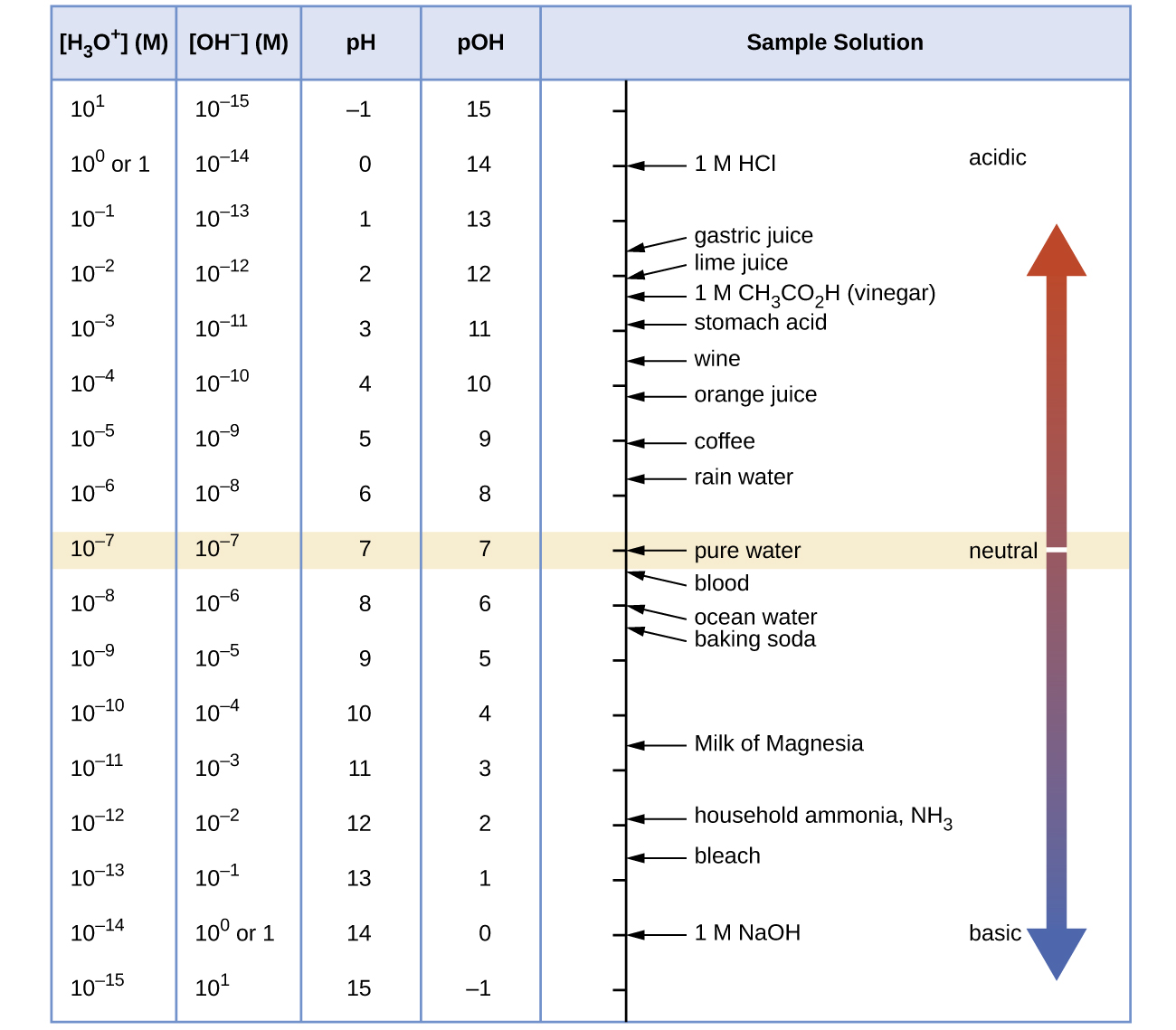
Calculate the hydrogen ion concentration and the hydroxide ion concentration in wine from its pH. See below Figure for useful information.
Q14.2.8
Calculate the hydronium ion concentration and the hydroxide ion concentration in lime juice from its pH. See Figure for useful information.
S14.2.9
[H3O+] = 1 × 10−2 M; [OH−] = 1 × 10−12 M
Q14.2.9
The hydronium ion concentration in a sample of rainwater is found to be 1.7 × 10−6 M at 25 °C. What is the concentration of hydroxide ions in the rainwater?
Q14.2.10
The hydroxide ion concentration in household ammonia is 3.2 × 10−3 M at 25 °C. What is the concentration of hydronium ions in the solution?
S14.2.10
[OH−] = 3.1 × 10−12 M
14.3: Relative Strengths of Acids and Bases
Q14.3.1
Explain why the neutralization reaction of a strong acid and a weak base gives a weakly acidic solution.
Q14.3.2
Explain why the neutralization reaction of a weak acid and a strong base gives a weakly basic solution.
The salt ionizes in solution, but the anion slightly reacts with water to form the weak acid. This reaction also forms OH−, which causes the solution to be basic.
Q14.3.3
Use this list of important industrial compounds (and Figure) to answer the following questions regarding: CaO, Ca(OH)2, CH3CO2H, CO2, HCl, H2CO3, HF, HNO2, HNO3, H3PO4, H2SO4, NH3, NaOH, Na2CO3.
- Identify the strong Brønsted-Lowry acids and strong Brønsted-Lowry bases.
- List those compounds in that can behave as Brønsted-Lowry acids with strengths lying between those of H3O+ and H2O.
- List those compounds in that can behave as Brønsted-Lowry bases with strengths lying between those of H2O and OH−.
Q14.3.4
The odor of vinegar is due to the presence of acetic acid, CH3CO2H, a weak acid. List, in order of descending concentration, all of the ionic and molecular species present in a 1-M aqueous solution of this acid.
S14.3.4
[H2O] > [CH3CO2H] > \(\ce{[H3O+]}\) ≈ \(\ce{[CH3CO2- ]}\) > [OH−]
Q14.3.5
Household ammonia is a solution of the weak base NH3 in water. List, in order of descending concentration, all of the ionic and molecular species present in a 1-M aqueous solution of this base.
Q14.3.4
Explain why the ionization constant, Ka, for H2SO4 is larger than the ionization constant for H2SO3.
S14.3.4
The oxidation state of the sulfur in H2SO4 is greater than the oxidation state of the sulfur in H2SO3.
Q14.3.7
Explain why the ionization constant, Ka, for HI is larger than the ionization constant for HF.
Q14.3.8
Gastric juice, the digestive fluid produced in the stomach, contains hydrochloric acid, HCl. Milk of Magnesia, a suspension of solid Mg(OH)2 in an aqueous medium, is sometimes used to neutralize excess stomach acid. Write a complete balanced equation for the neutralization reaction, and identify the conjugate acid-base pairs.
S14.3.8
\(\underset{\large\ce{BB}}{\ce{Mg(OH)2}(s)}+\underset{\large\ce{BA}}{\ce{HCl}(aq)}⟶\underset{\large\ce{CB}}{\ce{Mg^2+}(aq)}+\underset{\large\ce{CA}}{\ce{2Cl-}(aq)}+\underset{\:}{\ce{2H2O}(l)}\)
Q14.3.9
Nitric acid reacts with insoluble copper(II) oxide to form soluble copper(II) nitrate, Cu(NO3)2, a compound that has been used to prevent the growth of algae in swimming pools. Write the balanced chemical equation for the reaction of an aqueous solution of HNO3 with CuO.
Q14.3.10
What is the ionization constant at 25 °C for the weak acid \(\ce{CH3NH3+}\), the conjugate acid of the weak base CH3NH2, Kb = 4.4 × 10−4.
S14.3.10
\(K_\ce{a}=2.3×10^{−11}\)
Q14.3.11
What is the ionization constant at 25 °C for the weak acid \(\ce{(CH3)2NH2+}\), the conjugate acid of the weak base (CH3)2NH, Kb = 7.4 × 10−4?
Q14.3.12
Which base, CH3NH2 or (CH3)2NH, is the strongest base? Which conjugate acid, \(\ce{(CH3)2NH2+}\) or (CH3)2NH, is the strongest acid?
S14.3.12
The strongest base or strongest acid is the one with the larger Kb or Ka, respectively. In these two examples, they are (CH3)2NH and \(\ce{CH3NH3+}\).
Q14.3.3
Which is the stronger acid, \(\ce{NH4+}\) or HBrO?
Q14.3.14
Which is the stronger base, (CH3)3N or \(\ce{H2BO3-}\)?
S14.3.14
triethylamine.
Q14.3.15
Predict which acid in each of the following pairs is the stronger and explain your reasoning for each.
- H2O or HF
- B(OH)3 or Al(OH)3
- \(\ce{HSO3-}\) or \(\ce{HSO4-}\)
- NH3 or H2S
- H2O or H2Te
Q14.3.16
Predict which compound in each of the following pairs of compounds is more acidic and explain your reasoning for each.
- \(\ce{HSO4-}\) or \(\ce{HSeO4-}\)
- NH3 or H2O
- PH3 or HI
- NH3 or PH3
- H2S or HBr
S14.3.16
- \(\ce{HSO4-}\); higher electronegativity of the central ion. H2O;
- NH3 is a base and water is neutral, or decide on the basis of Ka values. HI;
- PH3 is weaker than HCl; HCl is weaker than HI. Thus, PH3 is weaker than HI.
- PH3; in binary compounds of hydrogen with nonmetals, the acidity increases for the element lower in a group.
- HBr; in a period, the acidity increases from left to right; in a group, it increases from top to bottom. Br is to the left and below S, so HBr is the stronger acid.
Q14.3.17
Rank the compounds in each of the following groups in order of increasing acidity or basicity, as indicated, and explain the order you assign.
- acidity: HCl, HBr, HI
- basicity: H2O, OH−, H−, Cl−
- basicity: Mg(OH)2, Si(OH)4, ClO3(OH) (Hint: Formula could also be written as HClO4).
- acidity: HF, H2O, NH3, CH4
Q14.3.18
Rank the compounds in each of the following groups in order of increasing acidity or basicity, as indicated, and explain the order you assign.
- acidity: NaHSO3, NaHSeO3, NaHSO4
- basicity: \(\ce{BrO2-}\), \(\ce{ClO2-}\), \(\ce{IO2-}\)
- acidity: HOCl, HOBr, HOI
- acidity: HOCl, HOClO, HOClO2, HOClO3
- basicity: \(\ce{NH2-}\), HS−, HTe−, \(\ce{PH2-}\)
- basicity: BrO−, \(\ce{BrO2-}\), \(\ce{BrO3-}\), \(\ce{BrO4-}\)
S14.3.18
- NaHSeO3 < NaHSO3 < NaHSO4; in polyoxy acids, the more electronegative central element—S, in this case—forms the stronger acid. The larger number of oxygen atoms on the central atom (giving it a higher oxidation state) also creates a greater release of hydrogen atoms, resulting in a stronger acid. As a salt, the acidity increases in the same manner.
- \(\ce{ClO2- < BrO2- < IO2-}\); the basicity of the anions in a series of acids will be the opposite of the acidity in their oxyacids. The acidity increases as the electronegativity of the central atom increases. Cl is more electronegative than Br, and I is the least electronegative of the three.
- HOI < HOBr < HOCl; in a series of the same form of oxyacids, the acidity increases as the electronegativity of the central atom increases. Cl is more electronegative than Br, and I is the least electronegative of the three.
- HOCl < HOClO < HOClO2 < HOClO3; in a series of oxyacids of the same central element, the acidity increases as the number of oxygen atoms increases (or as the oxidation state of the central atom increases).
- \(\ce{HTe- < HS- << PH2- < NH2-}\); \(\ce{PH2-}\) and \(\ce{NH2-}\) are anions of weak bases, so they act as strong bases toward H+. \(\ce{HTe-}\) and HS− are anions of weak acids, so they have less basic character. In a periodic group, the more electronegative element has the more basic anion.
- \(\ce{BrO4- < BrO3- < BrO2- < BrO-}\); with a larger number of oxygen atoms (that is, as the oxidation state of the central ion increases), the corresponding acid becomes more acidic and the anion consequently less basic.
Q14.3.19
Both HF and HCN ionize in water to a limited extent. Which of the conjugate bases, F− or CN−, is the stronger base? See Table.
Q14.3.20
The active ingredient formed by aspirin in the body is salicylic acid, C6H4OH(CO2H). The carboxyl group (−CO2H) acts as a weak acid. The phenol group (an OH group bonded to an aromatic ring) also acts as an acid but a much weaker acid. List, in order of descending concentration, all of the ionic and molecular species present in a 0.001-M aqueous solution of C6H4OH(CO2H).
\(\ce{[H2O] > [C6H4OH(CO2H)] > [H+]0 > [C6H4OH(CO2)- ] ≫ [C6H4O(CO2H)- ] > [OH- ]}\)
What do we represent when we write:
\[\ce{CH3CO2H}(aq)+\ce{H2O}(l)⇌\ce{H3O+}(aq)+\ce{CH3CO2-}(aq)?\]
Q14.3.21
Explain why equilibrium calculations are not necessary to determine ionic concentrations in solutions of certain strong electrolytes such as NaOH and HCl. Under what conditions are equilibrium calculations necessary as part of the determination of the concentrations of all ions of some other strong electrolytes in solution?
S14.3.21
Strong electrolytes are 100% ionized, and, as long as the component ions are neither weak acids nor weak bases, the ionic species present result from the dissociation of the strong electrolyte. Equilibrium calculations are necessary when one (or more) of the ions is a weak acid or a weak base.
Q14.3.22
Are the concentrations of hydronium ion and hydroxide ion in a solution of an acid or a base in water directly proportional or inversely proportional? Explain your answer.
Q14.3.23
What two common assumptions can simplify calculation of equilibrium concentrations in a solution of a weak acid?
S14.3.23
- Assume that the change in initial concentration of the acid as the equilibrium is established can be neglected, so this concentration can be assumed constant and equal to the initial value of the total acid concentration.
- Assume we can neglect the contribution of water to the equilibrium concentration of H3O+.
Q14.3.24
What two common assumptions can simplify calculation of equilibrium concentrations in a solution of a weak base?
Q14.3.25
Which of the following will increase the percent of NH3 that is converted to the ammonium ion in water (Hint: Use LeChâtelier’s principle.)?
- addition of NaOH
- addition of HCl
- addition of NH4Cl
S14.3.25
The addition of HCl
Q14.3.26
Which of the following will increase the percent of HF that is converted to the fluoride ion in water?
- addition of NaOH
- addition of HCl
- addition of NaF
Q14.3.27
What is the effect on the concentrations of \(\ce{NO2-}\), HNO2, and OH− when the following are added to a solution of KNO2 in water:
- HCl
- HNO2
- NaOH
- NaCl
- KNO
The equation for the equilibrium is:
\[\ce{NO2-}(aq)+\ce{H2O}(l)⇌\ce{HNO2}(aq)+\ce{OH-}(aq)\]S14.3.27
- Adding HCl will add H3O+ ions, which will then react with the OH− ions, lowering their concentration. The equilibrium will shift to the right, increasing the concentration of HNO2, and decreasing the concentration of \(\ce{NO2-}\) ions.
- Adding HNO2 increases the concentration of HNO2 and shifts the equilibrium to the left, increasing the concentration of \(\ce{NO2-}\) ions and decreasing the concentration of OH− ions.
- Adding NaOH adds OH− ions, which shifts the equilibrium to the left, increasing the concentration of \(\ce{NO2-}\) ions and decreasing the concentrations of HNO2.
- Adding NaCl has no effect on the concentrations of the ions.
- Adding KNO2 adds \(\ce{NO2-}\) ions and shifts the equilibrium to the right, increasing the HNO2 and OH− ion concentrations.
Q14.3.28
What is the effect on the concentration of hydrofluoric acid, hydronium ion, and fluoride ion when the following are added to separate solutions of hydrofluoric acid?
- HCl
- KF
- NaCl
- KOH
- HF
The equation for the equilibrium is:
\[\ce{HF}(aq)+\ce{H2O}(l)⇌\ce{H3O+}(aq)+\ce{F-}(aq)\]Q14.3.29
Why is the hydronium ion concentration in a solution that is 0.10 M in HCl and 0.10 M in HCOOH determined by the concentration of HCl?
S14.3.29
This is a case in which the solution contains a mixture of acids of different ionization strengths. In solution, the HCO2H exists primarily as HCO2H molecules because the ionization of the weak acid is suppressed by the strong acid. Therefore, the HCO2H contributes a negligible amount of hydronium ions to the solution. The stronger acid, HCl, is the dominant producer of hydronium ions because it is completely ionized. In such a solution, the stronger acid determines the concentration of hydronium ions, and the ionization of the weaker acid is fixed by the [H3O+] produced by the stronger acid.
Q14.3.30
From the equilibrium concentrations given, calculate Ka for each of the weak acids and Kb for each of the weak bases.
CH3CO2H: \(\ce{[H3O+]}\) = 1.34 × 10−3 M;
\(\ce{[CH3CO2- ]}\) = 1.34 × 10−3 M;[CH3CO2H] = 9.866 × 10−2 M;
ClO−: [OH−] = 4.0 × 10−4 M;
[HClO] = 2.38 × 10−5 M;
[ClO−] = 0.273 M;
HCO2H: [HCO2H] = 0.524 M;
\(\ce{[H3O+]}\) = 9.8 × 10−3 M; \(\ce{[HCO2- ]}\) = 9.8 × 10−3 M;\(\ce{C6H5NH3+ : [C6H5NH3+]}\) = 0.233 M;
[C6H5NH2] = 2.3 × 10−3 M;
\(\ce{[H3O+]}\) = 2.3 × 10−3 MFrom the equilibrium concentrations given, calculate Ka for each of the weak acids and Kb for each of the weak bases.
NH3: [OH−] = 3.1 × 10−3 M;
\(\ce{[NH4+]}\) = 3.1 × 10−3 M;[NH3] = 0.533 M;
HNO2: \(\ce{[H3O+]}\) = 0.011 M;
\(\ce{[NO2- ]}\) = 0.0438 M;[HNO2] = 1.07 M;
(CH3)3N: [(CH3)3N] = 0.25 M;
[(CH3)3NH+] = 4.3 × 10−3 M;[OH−] = 4.3 × 10−3 M;
\(\ce{NH4+ : [NH4+]}\) = 0.100 M;
[NH3] = 7.5 × 10−6 M;
[H3O+] = 7.5 × 10−6 M- \(K_\ce{b}=1.8×10^{−5};\)
- \(K_\ce{a}=4.5×10^{−4};\)
- \(K_\ce{b}=7.4×10^{−5};\)
- \(K_\ce{a}=5.6×10^{−10}\)
Q14.3.31
Determine Kb for the nitrite ion, \(\ce{NO2-}\). In a 0.10-M solution this base is 0.0015% ionized.
Q14.3.32
Determine Ka for hydrogen sulfate ion, \(\ce{HSO4-}\). In a 0.10-M solution the acid is 29% ionized.
S14.3.32
\(K_\ce{a}=1.2×10^{−2}\)
Q14.3.33
Calculate the ionization constant for each of the following acids or bases from the ionization constant of its conjugate base or conjugate acid:
- F−
- \(\ce{NH4+}\)
- \(\ce{AsO4^3-}\)
- \(\ce{(CH3)2NH2+}\)
- \(\ce{NO2-}\)
- \(\ce{HC2O4-}\) (as a base)
Q14.3.52
Calculate the ionization constant for each of the following acids or bases from the ionization constant of its conjugate base or conjugate acid:
- HTe− (as a base)
- \(\ce{(CH3)3NH+}\)
- \(\ce{HAsO4^3-}\) (as a base)
- \(\ce{HO2-}\) (as a base)
- \(\ce{C6H5NH3+}\)
- \(\ce{HSO3-}\) (as a base)
S14.3.52
- \(K_\ce{b}=4.3×10^{−12};\)
- \(K_\ce{a}=1.4×10^{−10};\)
- \(K_\ce{b}=1×10^{−7};\)
- \(K_\ce{b}=4.2×10^{−3};\)
- \(K_\ce{b}=4.2×10^{−3};\)
- \(K_\ce{b}=8.3×10^{−13}\)
Q14.3.53
For which of the following solutions must we consider the ionization of water when calculating the pH or pOH?
- 3 × 10−8 M HNO3
- 0.10 g HCl in 1.0 L of solution
- 0.00080 g NaOH in 0.50 L of solution
- 1 × 10−7 M Ca(OH)2
- 0.0245 M KNO3
Q14.3.54
Even though both NH3 and C6H5NH2 are weak bases, NH3 is a much stronger acid than C6H5NH2. Which of the following is correct at equilibrium for a solution that is initially 0.10 M in NH3 and 0.10 M in C6H5NH2?
- \(\ce{[OH- ]}=\ce{[NH4+]}\)
- \(\ce{[NH4+]}=\ce{[C6H5NH3+]}\)
- \(\ce{[OH- ]}=\ce{[C6H5NH3+]}\)
- [NH3] = [C6H5NH2]
- both a and b are correct
is the correct statement.
Q14.3.55
Calculate the equilibrium concentration of the nonionized acids and all ions in a solution that is 0.25 M in HCO2H and 0.10 M in HClO.
Q14.3.56
Calculate the equilibrium concentration of the nonionized acids and all ions in a solution that is 0.134 M in HNO2 and 0.120 M in HBrO.
S14.3.56
[H3O+] = 7.5 × 10−3 M
[HNO2] = 0.126 [OH−] = 1.3 × 10−12 M [BrO−] = 3.2 × 10−8 M [HBrO] = 0.120 MQ14.3.57
Calculate the equilibrium concentration of the nonionized bases and all ions in a solution that is 0.25 M in CH3NH2 and 0.10 M in C5H5N (Kb = 1.7 × 10−9).
Q14.3.58
Calculate the equilibrium concentration of the nonionized bases and all ions in a solution that is 0.115 M in NH3 and 0.100 M in C6H5NH2.
S14.3.58
[OH−] = \(\ce{[NO4+]}\) = 0.0014 M
[NH3] = 0.144 M [H3O+] = 6.9 × 10−12 M \(\ce{[C6H5NH3+]}\) = 3.9 × 10−8 M [C6H5NH2] = 0.100 MQ14.3.59
Using the Ka values in Appendix H, place \(\ce{Al(H2O)6^3+}\) in the correct location in Figure.
Q14.3.60
Calculate the concentration of all solute species in each of the following solutions of acids or bases. Assume that the ionization of water can be neglected, and show that the change in the initial concentrations can be neglected. Ionization constants can be found in Appendix H and Appendix I.
- 0.0092 M HClO, a weak acid
- 0.0784 M C6H5NH2, a weak base
- 0.0810 M HCN, a weak acid
- 0.11 M (CH3)3N, a weak base
- 0.120 M \(\ce{Fe(H2O)6^2+}\) a weak acid, Ka = 1.6 × 10−7
S14.3.60
\(\ce{\dfrac{[H3O+][ClO- ]}{[HClO]}}=\dfrac{(x)(x)}{(0.0092−x)}≈\dfrac{(x)(x)}{0.0092}=3.5×10^{−8}\)
Solving for x gives 1.79 × 10−5 M. This value is less than 5% of 0.0092, so the assumption that it can be neglected is valid. Thus, the concentrations of solute species at equilibrium are:
[H3O+] = [ClO] = 1.8 × 10−5 M[HClO] = 0.00092 M [OH−] = 5.6 × 10−10 M;
\(\ce{\dfrac{[C6H5NH3+][OH- ]}{[C6H5NH2]}}=\dfrac{(x)(x)}{(0.0784−x)}≈\dfrac{(x)(x)}{0.0784}=4.6×10^{−10}\)
Solving for x gives 6.01 × 10−6 M.
This value is less than 5% of 0.0784, so the assumption that it can be neglected is valid. Thus, the concentrations of solute species at equilibrium are: \(\ce{[CH3CO2- ]}\) = [OH−] = 6.0 × 10−6 M [C6H5NH2] = 0.00784 [H3O+] = 1.7× 10−9 M; \(\ce{\dfrac{[H3O+][CN- ]}{[HCN]}}=\dfrac{(x)(x)}{(0.0810−x)}≈\dfrac{(x)(x)}{0.0810}=4×10^{−10}\) Solving for x gives 5.69 × 10−6 M. This value is less than 5% of 0.0810, so the assumption that it can be neglected is valid. Thus, the concentrations of solute species at equilibrium are: [H3O+] = [CN−] = 5.7 × 10−6 M [HCN] = 0.0810 M [OH−] = 1.8 × 10−9 M; \(\ce{\dfrac{[(CH3)3NH+][OH- ]}{[(CH3)3N]}}=\dfrac{(x)(x)}{(0.11−x)}≈\dfrac{(x)(x)}{0.11}=7.4×10^{−5}\) Solving for x gives 2.85 × 10−3 M. This value is less than 5% of 0.11, so the assumption that it can be neglected is valid. Thus, the concentrations of solute species at equilibrium are: [(CH3)3NH+] = [OH−] = 2.9 × 10−3 M [(CH3)3N] = 0.11 M [H3O+] = 3.5 × 10−12 M; \(\ce{\dfrac{[Fe(H2O)5(OH)+][H3O+]}{[Fe(H2O)6^2+]}}=\dfrac{(x)(x)}{(0.120−x)}≈\dfrac{(x)(x)}{0.120}=1.6×10^{−7}\) Solving for x gives 1.39 × 10−4 M. This value is less than 5% of 0.120, so the assumption that it can be neglected is valid. Thus, the concentrations of solute species at equilibrium are: [Fe(H2O)5(OH)+] = [H3O+] = 1.4 × 10−4 M \(\ce{[Fe(H2O)6^2+]}\) = 0.120 M [OH−] = 7.2 × 10−11 M
Q14.3.61
Propionic acid, C2H5CO2H (Ka = 1.34 × 10−5), is used in the manufacture of calcium propionate, a food preservative. What is the hydronium ion concentration in a 0.698-M solution of C2H5CO2H?
Q14.3.62
White vinegar is a 5.0% by mass solution of acetic acid in water. If the density of white vinegar is 1.007 g/cm3, what is the pH?
S14.3.62
pH = 2.41
Q14.3.63
The ionization constant of lactic acid, CH3CH(OH)CO2H, an acid found in the blood after strenuous exercise, is 1.36 × 10−4. If 20.0 g of lactic acid is used to make a solution with a volume of 1.00 L, what is the concentration of hydronium ion in the solution?
Q14.3.64
Nicotine, C10H14N2, is a base that will accept two protons (K1 = 7 × 10−7, K2 = 1.4 × 10−11). What is the concentration of each species present in a 0.050-M solution of nicotine?
S14.3.64
[C10H14N2] = 0.049 M
[C10H14N2H+] = 1.9 × 10−4 M \(\ce{[C10H14N2H2^2+]}\) = 1.4 × 10−11 M [OH−] = 1.9 × 10−4 M [H3O+] = 5.3 × 10−11 MQ14.3.65
The pH of a 0.20-M solution of HF is 1.92. Determine Ka for HF from these data.
Q14.3.66
The pH of a 0.15-M solution of \(\ce{HSO4-}\) is 1.43. Determine Ka for \(\ce{HSO4-}\) from these data.
S14.3.66
\(K_\ce{a}=1.2×10^{−2}\)
Q14.3.67
The pH of a 0.10-M solution of caffeine is 11.16. Determine Kb for caffeine from these data:
\(\ce{C8H10N4O2}(aq)+\ce{H2O}(l)⇌\ce{C8H10N4O2H+}(aq)+\ce{OH-}(aq)\)Q14.3.68
The pH of a solution of household ammonia, a 0.950 M solution of NH3, is 11.612. Determine Kb for NH3 from these data.
S14.3.68
\(K_\ce{b}=1.77×10^{−5}\)
14.4: Hydrolysis of Salt Solutions
Q14.4.1
Determine whether aqueous solutions of the following salts are acidic, basic, or neutral:
- Al(NO3)3
- RbI
- KHCO2
- CH3NH3Br
Q14.4.2
Determine whether aqueous solutions of the following salts are acidic, basic, or neutral:
- FeCl3
- K2CO3
- NH4Br
- KClO4
S14.4.2
acidic; basic; acidic; neutral
Q14.4.3
Novocaine, C13H21O2N2Cl, is the salt of the base procaine and hydrochloric acid. The ionization constant for procaine is 7 × 10−6. Is a solution of novocaine acidic or basic? What are [H3O+], [OH−], and pH of a 2.0% solution by mass of novocaine, assuming that the density of the solution is 1.0 g/mL.
14.5: Polyprotic Acids
Q15.5.1
Which of the following concentrations would be practically equal in a calculation of the equilibrium concentrations in a 0.134-M solution of H2CO3, a diprotic acid:
- \(\ce{[H3O+]}\),
- \([OH^−]\)
- \([H_2CO_3]\)
- \(\ce{[HCO3- ]}\)
- \(\ce{[CO3^2- ]}\)
No calculations are needed to answer this question.
S15.5.1
[H3O+] and \(\ce{[HCO3- ]}\) are equal, H3O+ and \(\ce{HCO3-}\) are practically equal
Q15.5.2
Calculate the concentration of each species present in a 0.050-M solution of H2S.
Q15.5.3
Calculate the concentration of each species present in a 0.010-M solution of phthalic acid, C6H4(CO2H)2.
S15.5.3
\(\ce{C6H4(CO2H)2}(aq)+\ce{H2O}(l)⇌\ce{H3O+}(aq)+\ce{C6H4(CO2H)(CO2)-}(aq) \hspace{20px} K_\ce{a}=1.1×10^{−3}\)
\(\ce{C6H4(CO2H)(CO2)}(aq)+\ce{H2O}(l)⇌\ce{H3O+}(aq)+\ce{C6H4(CO2)2^2-}(aq) \hspace{20px} K_\ce{a}=3.9×10^{−6}\)
[C6H4(CO2H)2] 7.2 × 10−3 M, [C6H4(CO2H)(CO2)−] = [H3O+] 2.8 × 10−3 M, \(\ce{[C6H4(CO2)2^2- ]}\)3.9 × 10−6 M, [OH−] 3.6 × 10−12 M
Q15.5.4
Salicylic acid, HOC6H4CO2H, and its derivatives have been used as pain relievers for a long time. Salicylic acid occurs in small amounts in the leaves, bark, and roots of some vegetation (most notably historically in the bark of the willow tree). Extracts of these plants have been used as medications for centuries. The acid was first isolated in the laboratory in 1838.
- Both functional groups of salicylic acid ionize in water, with Ka = 1.0 × 10−3 for the—CO2H group and 4.2 × 10−13 for the −OH group. What is the pH of a saturated solution of the acid (solubility = 1.8 g/L).
- Aspirin was discovered as a result of efforts to produce a derivative of salicylic acid that would not be irritating to the stomach lining. Aspirin is acetylsalicylic acid, CH3CO2C6H4CO2H. The −CO2H functional group is still present, but its acidity is reduced, Ka = 3.0 × 10−4. What is the pH of a solution of aspirin with the same concentration as a saturated solution of salicylic acid (See Part a).
- Under some conditions, aspirin reacts with water and forms a solution of salicylic acid and acetic acid:
\[\ce{CH3CO2C6H4CO2H}(aq)+\ce{H2O}(l)⟶\ce{HOC6H4CO2H}(aq)+\ce{CH3CO2H}(aq)\]
- Which of the acids salicylic acid or acetic acid produces more hydronium ions in solution such a solution?
- What are the concentrations of molecules and ions in a solution produced by the hydrolysis of 0.50 g of aspirin dissolved in enough water to give 75 mL of solution?
Q15.5.5
The ion HTe− is an amphiprotic species; it can act as either an acid or a base.
- What is Ka for the acid reaction of HTe− with H2O?
- What is Kb for the reaction in which HTe− functions as a base in water?
- Demonstrate whether or not the second ionization of H2Te can be neglected in the calculation of [HTe−] in a 0.10 M solution of H2Te.
S15.5.5
- \(K_{\ce a2}=1×10^{−5};\)
- \(K_\ce{b}=4.3×10^{−12};\)
- \(\ce{\dfrac{[Te^2- ][H3O+]}{[HTe- ]}}=\dfrac{(x)(0.0141+x)}{(0.0141−x)}≈\dfrac{(x)(0.0141)}{0.0141}=1×10^{−5}\). Solving for x gives 1 × 10−5 M. Therefore, compared with 0.014 M, this value is negligible (0.071%).
14.6: Buffers
Q14.6.1
Explain why a buffer can be prepared from a mixture of NH4Cl and NaOH but not from NH3 and NaOH.
Q14.6.2
Explain why the pH does not change significantly when a small amount of an acid or a base is added to a solution that contains equal amounts of the acid H3PO4 and a salt of its conjugate base NaH2PO4.
S14.6.2
Excess H3O+ is removed primarily by the reaction:
\(\ce{H3O+}(aq)+\ce{H2PO4-}(aq)⟶\ce{H3PO4}(aq)+\ce{H2O}(l)\) Excess base is removed by the reaction: \(\ce{OH-}(aq)+\ce{H3PO4}(aq)⟶\ce{H2PO4-}(aq)+\ce{H2O}(l)\)
Q14.6.3
Explain why the pH does not change significantly when a small amount of an acid or a base is added to a solution that contains equal amounts of the base NH3 and a salt of its conjugate acid NH4Cl.
Q14.6.4
What is [H3O+] in a solution of 0.25 M CH3CO2H and 0.030 M NaCH3CO2?
\(\ce{CH3CO2H}(aq)+\ce{H2O}(l)⇌\ce{H3O+}(aq)+\ce{CH3CO2-}(aq) \hspace{20px} K_\ce{a}=1.8×10^{−5}\)
S14.6.4
[H3O+] = 1.5 × 10−4 M
Q14.6.5
What is [H3O+] in a solution of 0.075 M HNO2 and 0.030 M NaNO2?
S14.6.6
\(\ce{HNO2}(aq)+\ce{H2O}(l)⇌\ce{H3O+}(aq)+\ce{NO2-}(aq) \hspace{20px} K_\ce{a}=4.5×10^{−5}\)Q14.6.6
What is [OH−] in a solution of 0.125 M CH3NH2 and 0.130 M CH3NH3Cl?
S14.6.6
\(\ce{CH3NH2}(aq)+\ce{H2O}(l)⇌\ce{CH3NH3+}(aq)+\ce{OH-}(aq) \hspace{20px} K_\ce{b}=4.4×10^{−4}\)[OH−] = 4.2 × 10−4 M
Q14.6.7
What is [OH−] in a solution of 1.25 M NH3 and 0.78 M NH4NO3?
S14.6.7
\(\ce{NH3}(aq)+\ce{H2O}(l)⇌\ce{NH4+}(aq)+\ce{OH-}(aq) \hspace{20px} K_\ce{b}=1.8×10^{−5}\)Q14.6.8
What concentration of NH4NO3 is required to make [OH−] = 1.0 × 10−5 in a 0.200-M solution of NH3?
S14.6.8
[NH4NO3] = 0.36 M
Q14.6.9A
What concentration of NaF is required to make [H3O+] = 2.3 × 10−4 in a 0.300-M solution of HF?
Q14.6.9B
What is the effect on the concentration of acetic acid, hydronium ion, and acetate ion when the following are added to an acidic buffer solution of equal concentrations of acetic acid and sodium acetate:
- HCl
- KCH3CO2
- NaCl
- KOH
- CH3CO2H
S14.6.10
- The added HCl will increase the concentration of H3O+ slightly, which will react with \(\ce{CH3CO2-}\) and produce CH3CO2H in the process. Thus, \(\ce{[CH3CO2- ]}\) decreases and [CH3CO2H] increases.
- The added KCH3CO2 will increase the concentration of \(\ce{[CH3CO2- ]}\) which will react with H3O+ and produce CH3CO2 H in the process. Thus, [H3O+] decreases slightly and [CH3CO2H] increases.
- The added NaCl will have no effect on the concentration of the ions.
- The added KOH will produce OH− ions, which will react with the H3O+, thus reducing [H3O+]. Some additional CH3CO2H will dissociate, producing \(\ce{[CH3CO2- ]}\) ions in the process. Thus, [CH3CO2H] decreases slightly and \(\ce{[CH3CO2- ]}\) increases.
- The added CH3CO2H will increase its concentration, causing more of it to dissociate and producing more \(\ce{[CH3CO2- ]}\) and H3O+ in the process. Thus, [H3O+] increases slightly and \(\ce{[CH3CO2- ]}\) increases.
Q14.6.11
What is the effect on the concentration of ammonia, hydroxide ion, and ammonium ion when the following are added to a basic buffer solution of equal concentrations of ammonia and ammonium nitrate:
- KI
- NH3
- HI
- NaOH
- NH4Cl
What will be the pH of a buffer solution prepared from 0.20 mol NH3, 0.40 mol NH4NO3, and just enough water to give 1.00 L of solution?
pH = 8.95
Calculate the pH of a buffer solution prepared from 0.155 mol of phosphoric acid, 0.250 mole of KH2PO4, and enough water to make 0.500 L of solution.
How much solid NaCH3CO2•3H2O must be added to 0.300 L of a 0.50-M acetic acid solution to give a buffer with a pH of 5.00? (Hint: Assume a negligible change in volume as the solid is added.)
37 g (0.27 mol)
What mass of NH4Cl must be added to 0.750 L of a 0.100-M solution of NH3 to give a buffer solution with a pH of 9.26? (Hint: Assume a negligible change in volume as the solid is added.)
Q14.6.1
A buffer solution is prepared from equal volumes of 0.200 M acetic acid and 0.600 M sodium acetate. Use 1.80 × 10−5 as Ka for acetic acid.
- What is the pH of the solution?
- Is the solution acidic or basic?
Q14.6.1
What is the pH of a solution that results when 3.00 mL of 0.034 M HCl is added to 0.200 L of the original buffer?
- pH = 5.222;
- The solution is acidic. (c) pH = 5.221
Q14.6.1
A 5.36–g sample of NH4Cl was added to 25.0 mL of 1.00 M NaOH and the resulting solution diluted to 0.100 L.
- What is the pH of this buffer solution?
- Is the solution acidic or basic?
- What is the pH of a solution that results when 3.00 mL of 0.034 M HCl is added to the solution?
Which acid in [link] is most appropriate for preparation of a buffer solution with a pH of 3.1? Explain your choice.
To prepare the best buffer for a weak acid HA and its salt, the ratio \(\dfrac{\ce{[H3O+]}}{K_\ce{a}}\) should be as close to 1 as possible for effective buffer action. The [H3O+] concentration in a buffer of pH 3.1 is [H3O+] = 10−3.1 = 7.94 × 10−4 M
We can now solve for Ka of the best acid as follows:\(\dfrac{\ce{[H3O+]}}{K_\ce{a}}=1\)
\(K_\ce{a}=\dfrac{\ce{[H3O+]}}{1}=7.94×10^{−4}\)
In [link], the acid with the closest Ka to 7.94 × 10−4 is HF, with a Ka of 7.2 × 10−4.Which acid in [link] is most appropriate for preparation of a buffer solution with a pH of 3.7? Explain your choice.
Which base in [link] is most appropriate for preparation of a buffer solution with a pH of 10.65? Explain your choice.
For buffers with pHs > 7, you should use a weak base and its salt. The most effective buffer will have a ratio \(\dfrac{\ce{[OH- ]}}{K_\ce{b}}\) that is as close to 1 as possible. The pOH of the buffer is 14.00 − 10.65 = 3.35. Therefore, [OH−] is [OH−] = 10−pOH = 10−3.35 = 4.467 × 10−4 M.
We can now solve for Kb of the best base as follows: \(\dfrac{\ce{[OH- ]}}{K_\ce{b}}=1\) Kb = [OH−] = 4.47 × 10−4 In [link], the base with the closest Kb to 4.47 × 10−4 is CH3NH2, with a Kb = 4.4 × 10−4.Which base in [link] is most appropriate for preparation of a buffer solution with a pH of 9.20? Explain your choice.
Q14.6.4
Saccharin, C7H4NSO3H, is a weak acid (Ka = 2.1 × 10−2). If 0.250 L of diet cola with a buffered pH of 5.48 was prepared from 2.00 × 10−3 g of sodium saccharide, Na(C7H4NSO3), what are the final concentrations of saccharine and sodium saccharide in the solution?
S14.6.4
The molar mass of sodium saccharide is 205.169 g/mol. Using the abbreviations HA for saccharin and NaA for sodium saccharide the number of moles of NaA in the solution is:
9.75 × 10−6 mol. This ionizes initially to form saccharin ions, A−, with: [A−] = 3.9 × 10−5 MQ14.6.5
What is the pH of 1.000 L of a solution of 100.0 g of glutamic acid (C5H9NO4, a diprotic acid; K1 = 8.5 × 10−5, K2 = 3.39 × 10−10) to which has been added 20.0 g of NaOH during the preparation of monosodium glutamate, the flavoring agent? What is the pH when exactly 1 mol of NaOH per mole of acid has been added?
14.7: Acid-Base Titrations
Q14.7.1
Explain how to choose the appropriate acid-base indicator for the titration of a weak base with a strong acid.
S14.7.1
At the equivalence point in the titration of a weak base with a strong acid, the resulting solution is slightly acidic due to the presence of the conjugate acid. Thus, pick an indicator that changes color in the acidic range and brackets the pH at the equivalence point. Methyl orange is a good example.
Q14.7.2
Explain why an acid-base indicator changes color over a range of pH values rather than at a specific pH.
Q14.7.3
Why can we ignore the contribution of water to the concentrations of H3O+ in the solutions of following acids:
- 0.0092 M HClO, a weak acid
- 0.0810 M HCN, a weak acid
- 0.120 M \(\ce{Fe(H2O)6^2+}\) a weak acid, Ka = 1.6 × 10−7
but not the contribution of water to the concentration of OH−?
S14.7.3
In an acid solution, the only source of OH− ions is water. We use Kw to calculate the concentration. If the contribution from water was neglected, the concentration of OH− would be zero.
Q14.7.4
We can ignore the contribution of water to the concentration of OH− in a solution of the following bases:
0.0784 M C6H5NH2, a weak base
0.11 M (CH3)3N, a weak base
but not the contribution of water to the concentration of H3O+?
Q14.7.5
Draw a curve for a series of solutions of HF. Plot [H3O+]total on the vertical axis and the total concentration of HF (the sum of the concentrations of both the ionized and nonionized HF molecules) on the horizontal axis. Let the total concentration of HF vary from 1 × 10−10 M to 1 × 10−2 M.
![A graph is shown that is titled “Plot of [ H subscript 3 O superscript + ] Against [ H F ].” The horizontal axis is labeled “[ H F ], M.” The axis begins at 10 superscript negative 10 and includes markings every 10 superscript 2 units up to 1.0. The vertical axis is labeled “[ H subscript 3 O superscript plus ], M” and begins at 10 superscript negative 10 and increases by 10 superscript 2 up to 1.0. A black curve starts at the left side of the graph at (10 superscript negative 10, 10 superscript negative 7). The line extends horizontally to a horizontal axis value of 10 superscript negative 8. After this, the line gradually increases at a steady rate to a value just over 10 superscript negative 3 at a horizontal axis value of 10 superscript negative 2.](http://cnx.org/resources/bbd3359c4d4d301b0f4471bd309310444779db56/CNX_Chem_14_07_Exercise5_img.jpg)
Q14.7.6
Draw a curve similar to that shown in Figure for a series of solutions of NH3. Plot [OH−] on the vertical axis and the total concentration of NH3 (both ionized and nonionized NH3 molecules) on the horizontal axis. Let the total concentration of NH3 vary from 1 × 10−10 M to 1 × 10−2 M.
Q14.7.7
Calculate the pH at the following points in a titration of 40 mL (0.040 L) of 0.100 M barbituric acid (Ka = 9.8 × 10−5) with 0.100 M KOH.
- no KOH added
- 20 mL of KOH solution added
- 39 mL of KOH solution added
- 40 mL of KOH solution added
- 41 mL of KOH solution added
S14.7.7
- pH = 2.50;
- pH = 4.01;
- pH = 5.60;
- pH = 8.35;
- pH = 11.08
Q14.7.8
The indicator dinitrophenol is an acid with a Ka of 1.1 × 10−4. In a 1.0 × 10−4-M solution, it is colorless in acid and yellow in base. Calculate the pH range over which it goes from 10% ionized (colorless) to 90% ionized (yellow).


