16.6: Additional examples of enzymatic hydride transfer reactions
- Page ID
- 1014
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)16.6A: More reactions involving nicotinamide adenine dinucleotide and flavin
Most reactions involving nicotinamide adenine dinucleotide and flavin coenzymes involve delivery of hydride to carbonyl, imine, or alkene groups (or acceptance of hydride from alcohols, amines, or alkanes). There are, however, exceptions. Recall, for example, the series of carbocation rearrangements that lead to the formation of squalene, a precursor to cholesterol, from two molecules of farnesyl diphosphate (section 15.9). The final step of this reaction, which we skipped in section 15.9 but now can examine in more detail, is the delivery of a hydride nucleophile from NADPH. This causes the opening of the cyclopropyl ring and quenching of the carbocation.
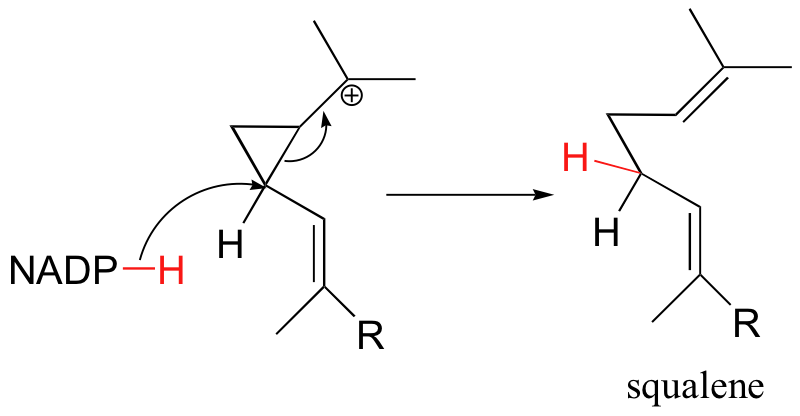
The requirement for NADPH in this reaction was exploited by researchers in an experiment designed to provide evidence for the proposed mechanism shown in section 15.9 (Chem. Eng. News, January 6, 1997, p. 6; J. Am. Chem. Soc. 1996, 118, 13089). They reasoned that if they could allow the reaction to start as usual, but then prevent the final NADPH-dependent reduction step from occurring, the reaction would become 'stalled' at the tertiary carbocation stage. Eventually, a water molecule might then make its way into the active site and attack the positively charged carbon.
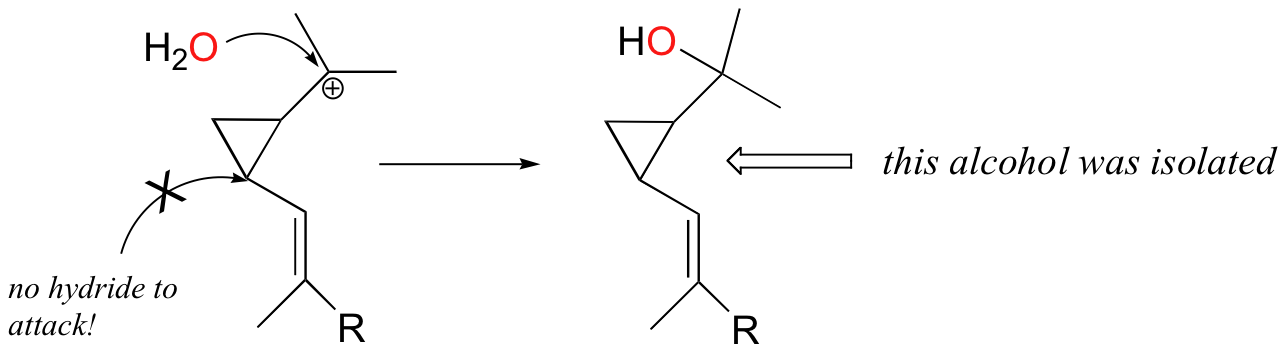
Isolation of the resulting tertiary cyclopropropylcarbinyl alcohol shown above- which is stable enough to isolate and characterize by 1H NMR and mass spectrometry - would provide convincing evidence for the existence of the proposed tertiary carbocation intermediate. This technique is known as 'trapping' an intermediate.
In order to prevent the hydride transfer from proceeding, the researchers first hydrogenated the isolated C2-C3 double bond on a sample of NADPH, using a palladium catalyst (under the conditions used, the conjugated C5-C6 double bond was left unreduced).
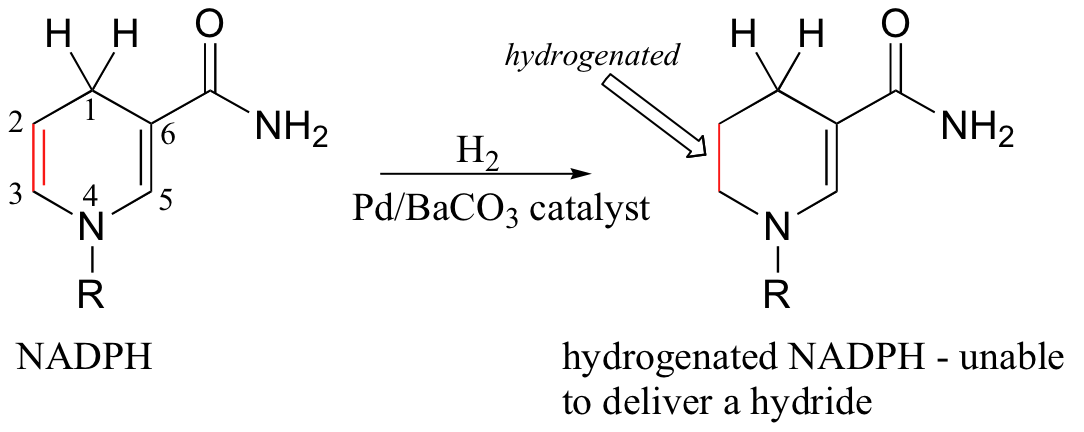
The resulting hydrogenated NADPH molecule bound well to the enzyme site normally occupied by normal NADPH, but was unable to deliver a hydride due to lack of the C2-C3 double bond. As hypothesized, the tertiary alcohol was found to have been produced in the altered reaction.
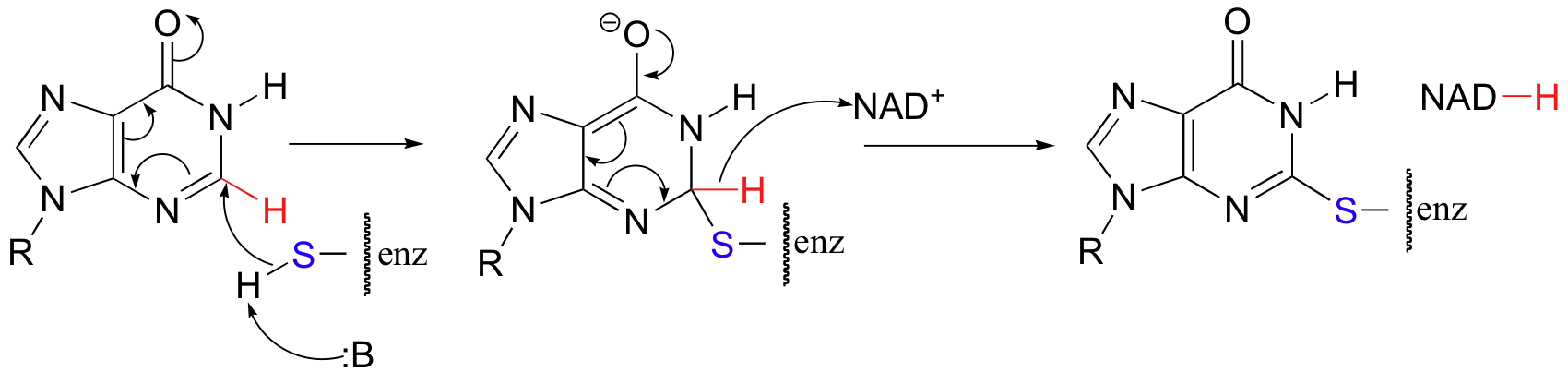
The guanine biosythetic pathway includes an interesting and unusual NAD+-dependent oxidative step that resembles a nucleophilic aromatic substitution (section 14.2B), but with a hydride 'leaving group'.
16.6B: Reactions with coenzymes derived from folic acid
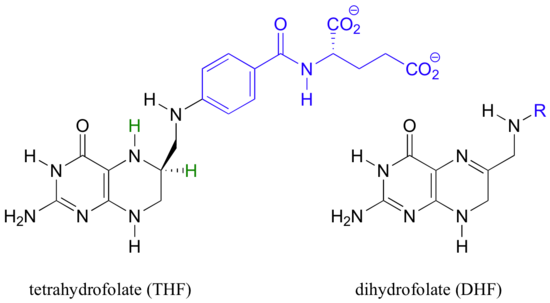
Folic acid is an essential vitamin in the human diet. Folic acid itself has no biologically activity, but several reduced folate derivatives play important roles in metabolism, all involving single carbon transfer reactions - and, more relevant to this chapter, hydride transfer steps.
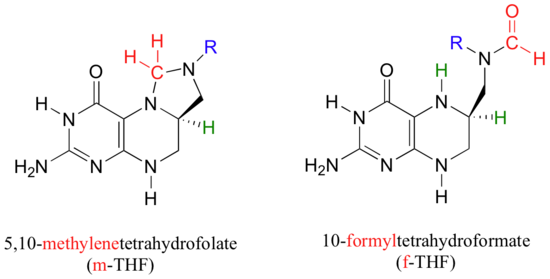
You may recall, from problem P12.14, how 10-formyl-THF plays the role of formyl group donor.
5,10-methylene-tetrahydrofolate (m-THF) is a coenzyme involved in the final step in the biosynthesis of the DNA base thymidine monophosphate, a reaction catalyzed by thymidylate synthase. In the course of this reaction, m-THF is converted to dihydrofolate (DHF), as deoxyuridine monophosphate (dUMP) gains a methyl group to become thymidine monophosphate.
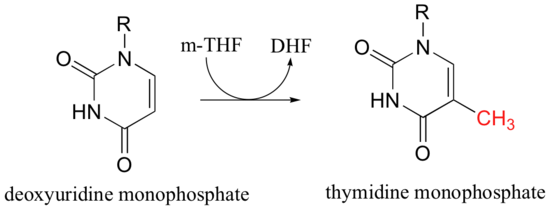
The reactions begins with the five-membered ring of m-THF breaking apart to create an imine intermediate (step 1a below). The imine becomes an electrophile in a Michael addition-like step (section 14.1, steps 1b and 2 below).
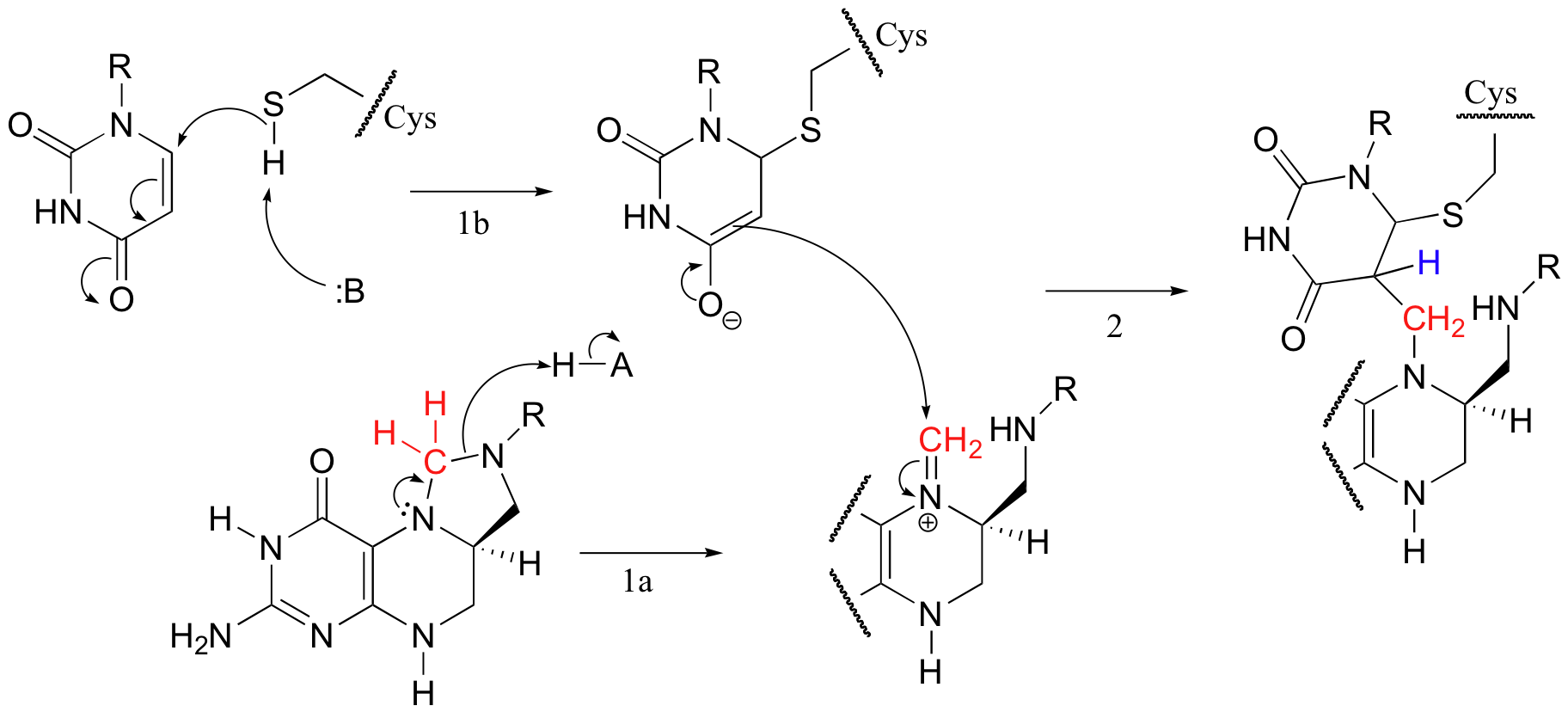
Next, tetrahydrofolate is eliminated in an E1cb mechanism (section 14.1, steps 3a and 3b below). Notice that this is where the single carbon (in red) is transferred from methyl-THF to dUMP.
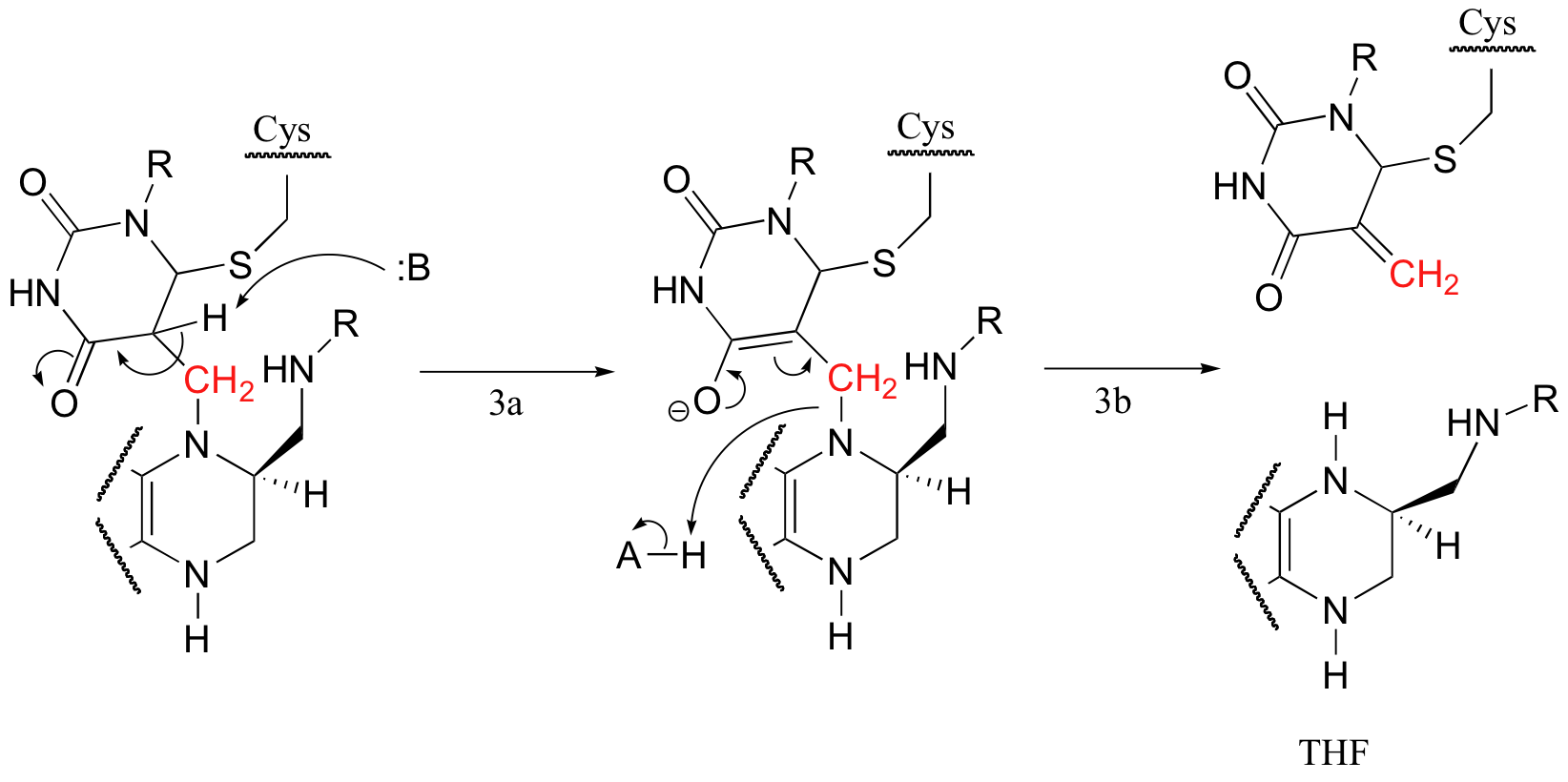
The final step in the mechanism is where it gets really interesting: a hydride ion is transferred from the tetrahydrofolate coenzyme to the methylene (CH2) group on the deoxynucleotide substrate. Essentially, this is a conjugated SN2' step (section 9.5) with hydride as the nucleophile and the active site cysteine as the leaving group.
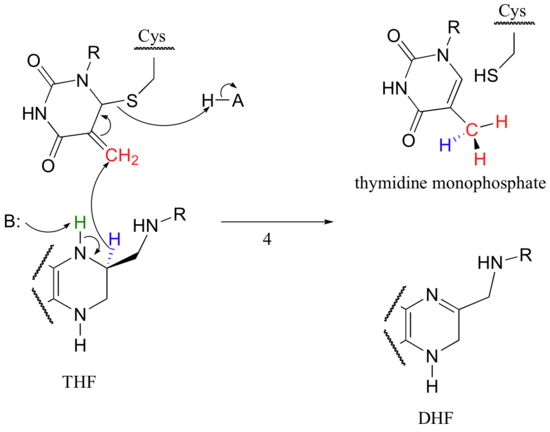
The dihydrofolate generated in this reaction is reduced back to tetrahydrofolate by an NADH-dependent enzyme called DHF reductase - this is simply an imine to amine hydrogenation (section 16.4D).
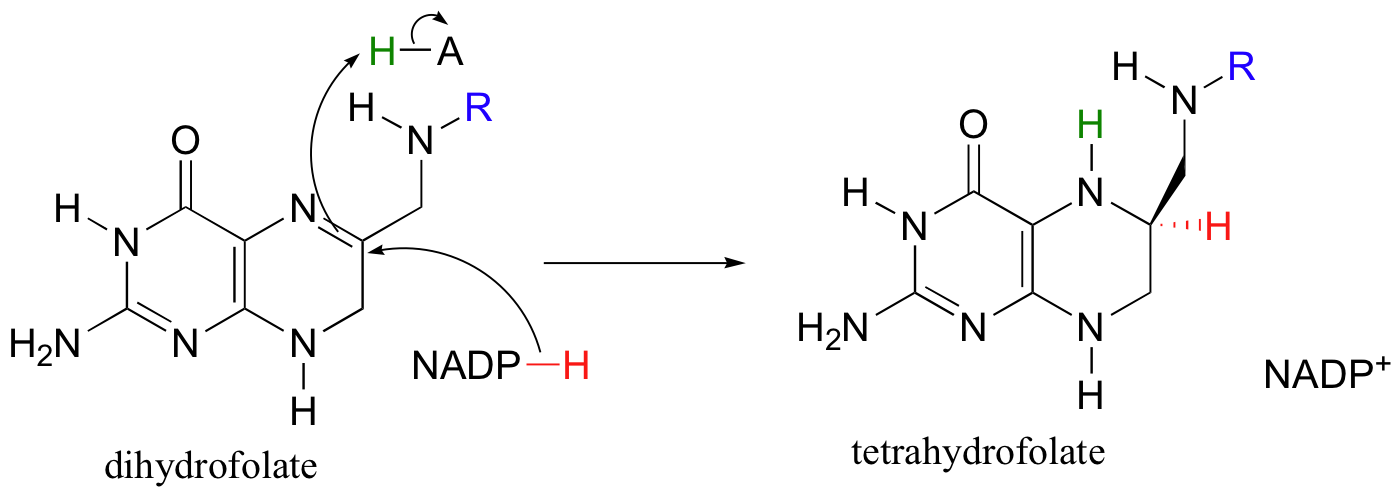
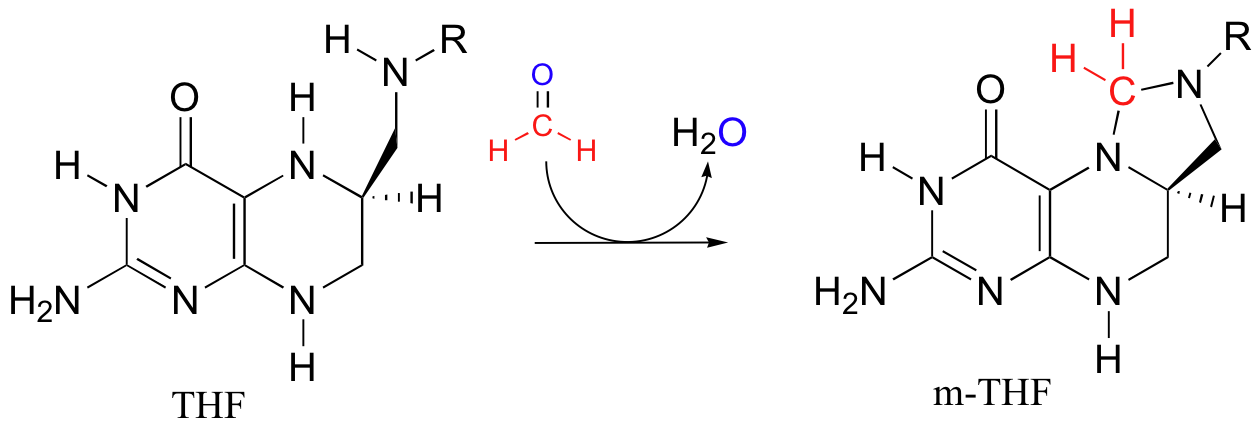
To complete the cycle, THF is converted back to methylene-THF when it 'absorbs' formaldehyde, which is created as a (potentially toxic) by-product of various other metabolic reactions such as that catalyzed by serine hydroxymethyltransferase (section 14.4D). We walked through the mechanism for the THF plus formaldehyde to methylene-THF reaction in section 11.6D.