11.6: Imine (Schiff base) formation
- Page ID
- 964
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)11.6A: Imines - the general picture
The electrophilic carbon atoms of aldehydes and ketones can be targets of nucleophilic attack by amines. The end result of this reaction is a compound in which the C=O double bond is replaced by a C=N double bond. This type of compound is known as an imine, or Schiff base.
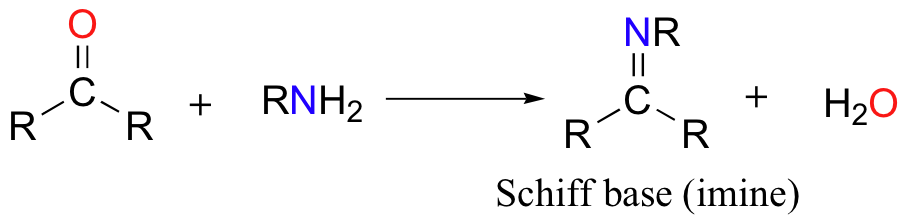
Mechanistically, the formation of an imine involves two steps. First, the amine nitrogen acts as a nucleophile, attacking the carbonyl carbon. This is closely analogous to hemiacetal and hemiketal formation.
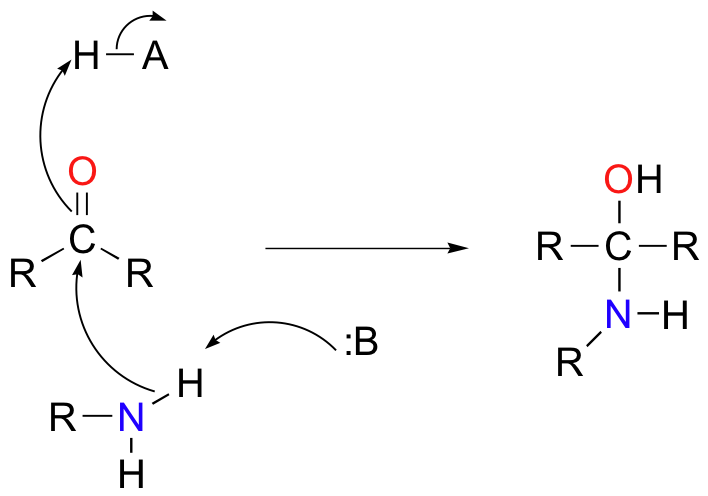
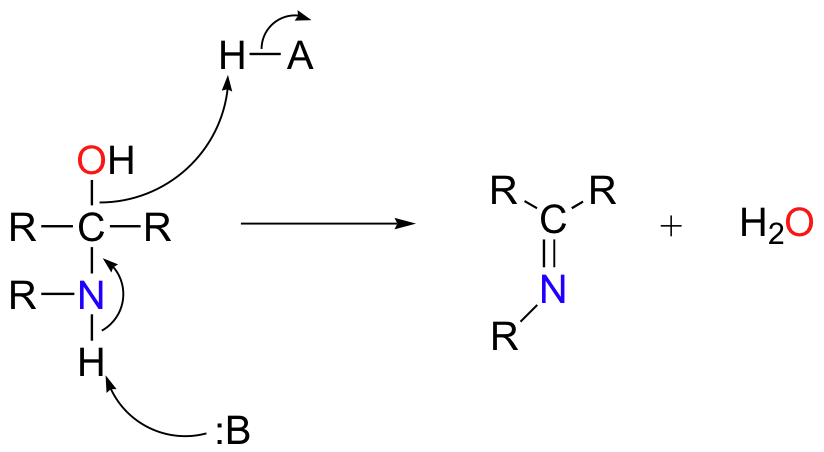
Based on your knowledge of the mechanism of acetal and ketal formation, you might expect that the next step would be attack by a second amine to form a compound with a carbon bound to two amine groups – the nitrogen version of a ketal. Instead, what happens next is that the nitrogen is deprotonated, and the electrons from this N-H bond ‘push’ the oxygen off of the carbon, leaving us with a C=N double bond (an imine) and a displaced water molecule.
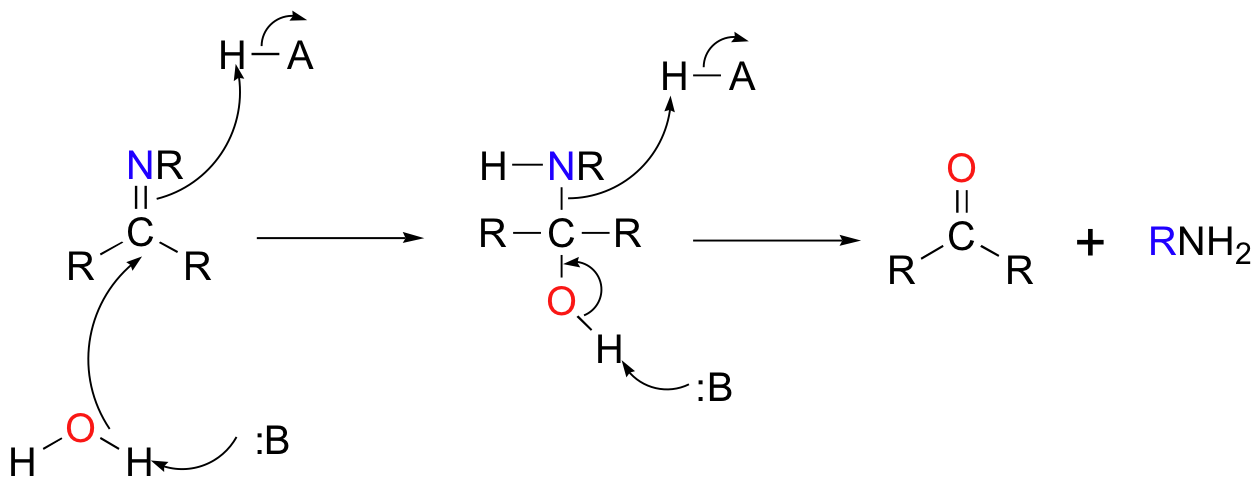
The conversion of an imine back to an aldehyde or ketone is a hydrolysis, and mechanistically is simply the reverse of imine formation:
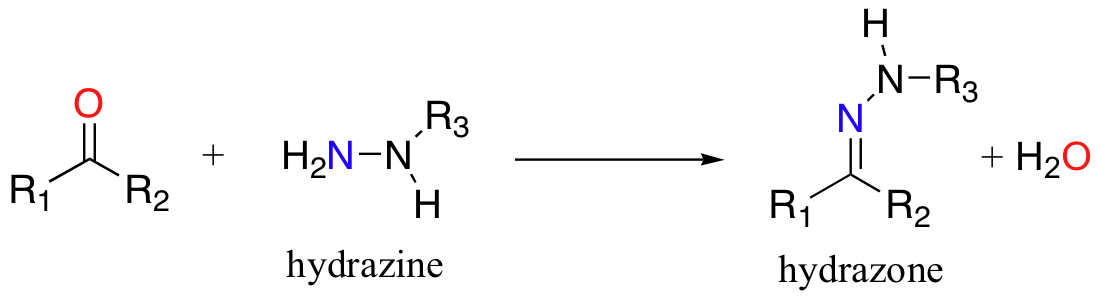
Hydrazones are close relatives to imines, but are not abundantly in biological molecules. Hydrazones are formed in reactions between aldehydes/ketones and hydrazines, a functional group containing a nitrogen-nitrogen bond.
Guanafuracin, a known antibiotic compound, is a hydrazone, and can be prepared easily by combining equimolar amounts of the appropriate aldehyde and hydrazine:
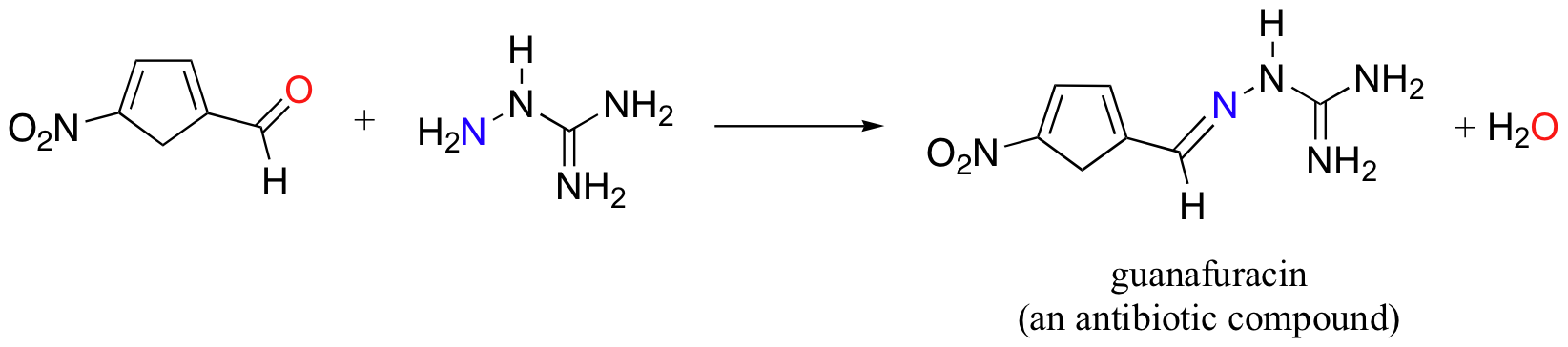
The mechanism for hydrazone formation is analogous to that of imine formation.
11.6B: Pyridoxal phosphate coenzyme links to enzymes by a Schiff base
Schiff base (imine) formation is a very important reaction in biological chemistry. One example involves the chemistry of pyridoxal phosphate (PLP), a derivative of pyridoxine, commonly known as vitamin B6.
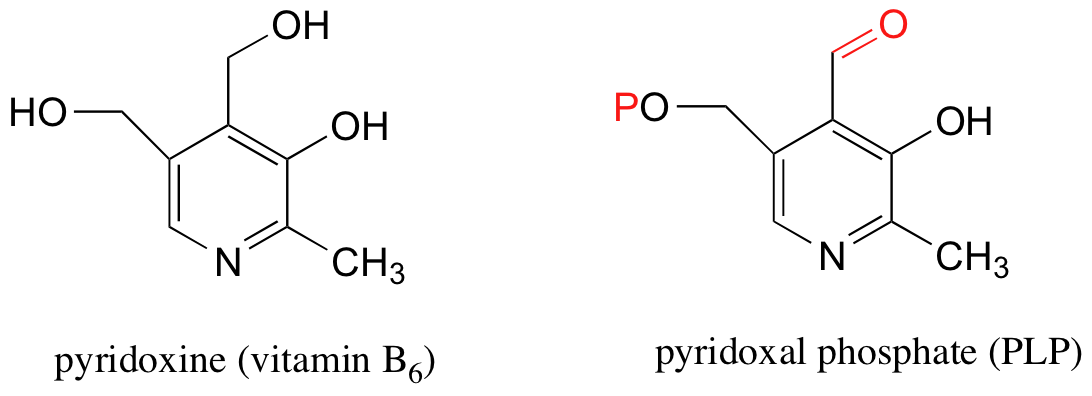
We will study the reactivity of PLP in more depth in section 14.4, but for know what you need to know is that PLP binds to a number of specific enzymes and plays a critical role in helping these enzymes to catalyze their reactions. Most enzymes that interact with PLP catalyze reactions involved in the metabolism of amino acids.
Notice that PLP has an aldehyde group. In many PLP-dependent enzymatic reactions, one of the first things that happens is that PLP forms a Schiff base link with a lysine residue on the enzyme.

Often, the next step is what could be called a Schiff base transfer: the PLP is transferred from the enzyme lysine to the nitrogen of the amino acid substrate.
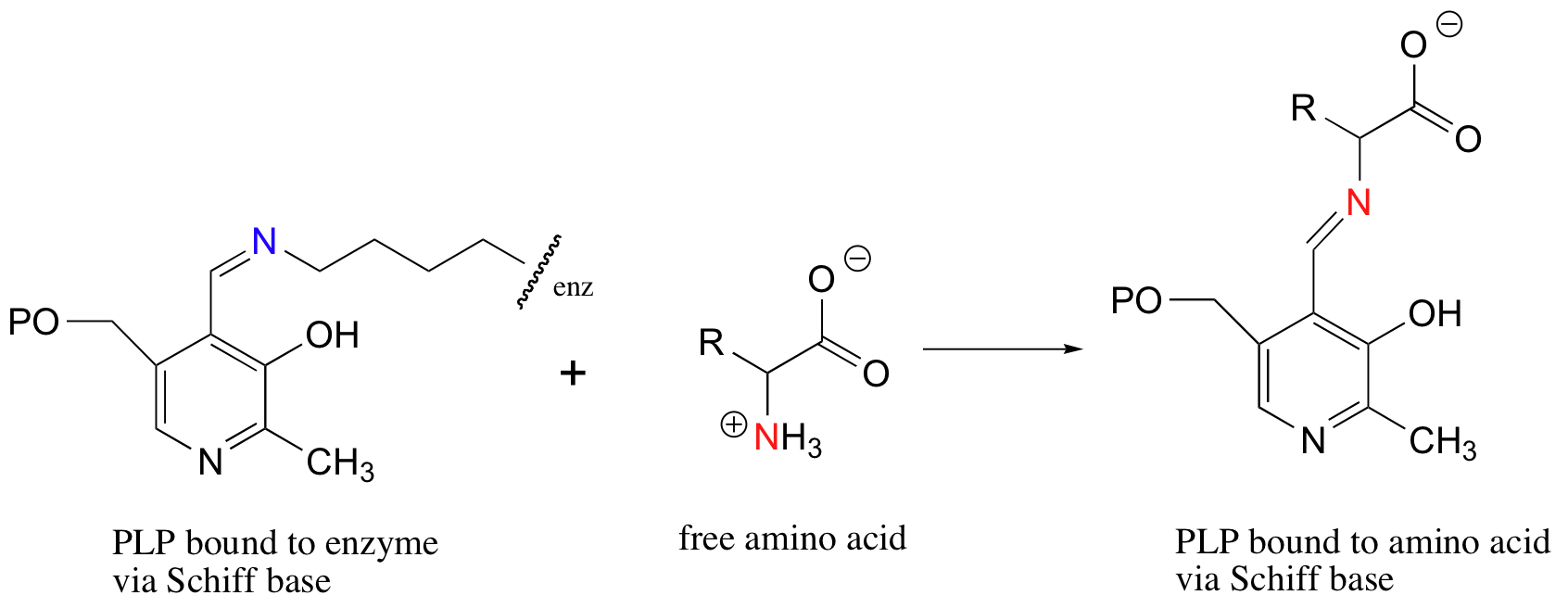
11.6C: Schiff base formation in aldolase reactions
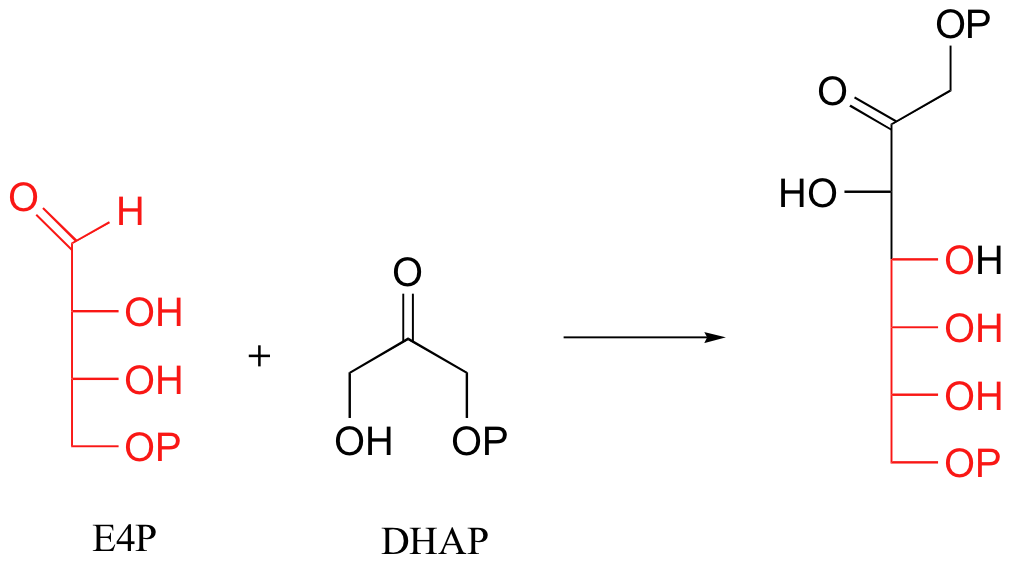
Another important example of Schiff base formation in biological chemistry involves carbon-carbon bond-forming reactions catalyzed by enzymes called aldolases (we will study these reactions in detail in section 13.3). In an aldol reaction, two carbonyl-containing compounds condense to form a single molecule. A key step in this process is the formation of a Schiff base between one of the reactants and a lysine in the active site of the enzyme. For example, when plants convert carbon in the form of CO2 into carbohydrate, one of the early reactions that takes place is the condensation of the four-carbon sugar erythrose-4-phosphate (E4P) with dihydroxyacetone phosphate (DHAP) to form the seven-carbon sugar sedoheptulose-1,7-bisphosphate:
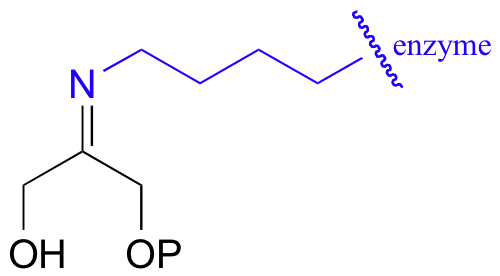
The DHAP substrate binds to the enzyme first, and forms a Schiff base with a specific active site lysine residue:
When we study this reaction in its entirety in section 13.3, and see how formation of the Schiff base is a critical part of the enzyme’s catalytic strategy.
11.6D: Tetrahydrofolate is a donor/acceptor of single-carbon groups
Tetrahydrofolate, a coenzyme that is derived from folic acid (one of the B vitamins), participates in an interesting variation on the acetal / Schiff base mechanistic pattern. Serine hydroxymethyltransferase (we'll study this reaction again in section 14.4D) catalyzes the reversible conversion of the amino acids glycine and serine:
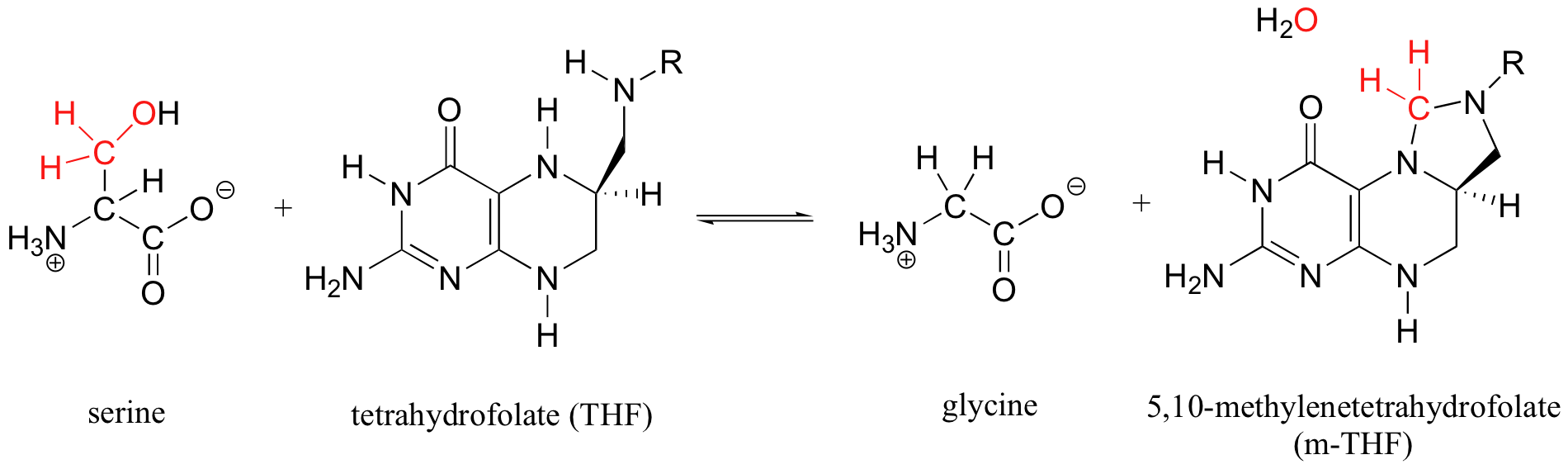
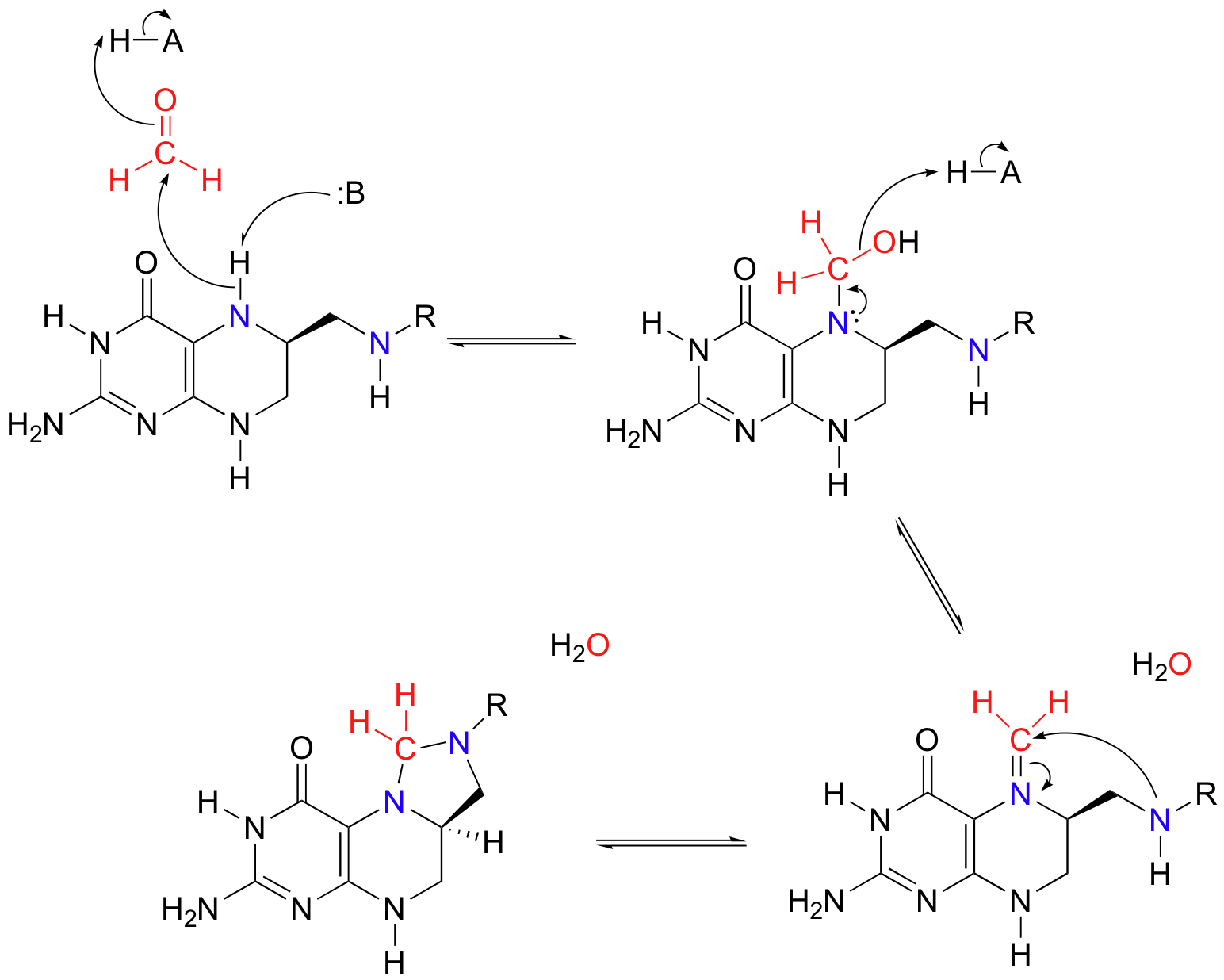
More than one mechanism has been proposed for this reaction, but one likely pathway involves free formaldehyde as an intermediate. In the serine to glycine direction, the formaldehyde intermediate is incorporated into tetrahydrofolate through the formation of what could be termed a 'cyclic nitrogen acetal' in the resulting 5,10-methylene-tetrahydrofolate. Notice that THF in this reaction serves as an acceptor of a single carbon group - in this case, formaldehyde.
Contributors
- Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)


