Extra Credit 34
- Page ID
- 82947
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.5.2
List some things that are typically considered when selecting a battery for a new application.
Answer 17.5.2:
Because batteries can be used in many different things it is important to look at how each part of the battery will affect the new application.
You will want to look at:
- cost (you don't want your product to be too expensive to produce)
- materials (what materials are best suited for oxidation/reduction in the conditions needed for the application)
-
the type of battery:
primary (which cannot be recharged) or secondary (which can be recharged) for more information on primary and secondary batteries visit this page.
- energy requirements, how does the application need to last (which depends on the potential from reactants to products the battery still has within its cell) and how much power does it use, this will be important for how many volts the battery should be
- mass (important for functionality and ability to transport)
- toxicity of materials and how to dispose of dead batteries (because batteries use many toxic materials, it is important to consider how to dispose of them and if it is possible to dispose of them safely) Toxic materials include acid, lead, nickel, cadmium, lithium, and mercury.
- access to materials (the type of material is important but the availability is also important if the product is going to be mass produced)
Q12.2.1
Describe the effect of each of the following on the rate of the reaction of magnesium metal with a solution of hydrochloric acid: the molarity of the hydrochloric acid, the temperature of the solution, and the size of the pieces of magnesium.
Answer 12.2.1:
Molarity: A higher molarity of hydrochloric acid will speed up the rate of the reaction. Molarity means how many moles of a substance in one liter. The more moles in one liter the higher the molarity. With a higher molarity, there will be more molecules hitting one another causing the rate of the reaction to speed up. If there is a lower molarity the rate of the reaction will slow because there will be fewer molecules in solution to collide with magnesium and create a reaction.
Temperature: An increase in temperature will increase the rate of a reaction while a decrease in temperature will decrease the rate of reaction. This happens because temperature affects how fast particles can move. At higher temperatures, molecules are able to move faster and have more kinetic energy. At lower temperatures, molecules move slower and have less kinetic energy. One way to think about this is to picture an ice cube, the water molecules in the ice have very little energy, they are in a solid state and cannot move as much. As the ice cube melts the water molecules are in a liquid state and have more energy and thus the ability to move around more. In a gaseous state, the most kinetic energy is with the water molecules. As the temperature goes up so does the energy of the molecules. More energetic molecules mean stronger collisions as well as more frequent collisions. These factors contribute to more chances of a reaction.
Size of Pieces: The smaller the size of the pieces of magnesium the faster the rate of the reaction. This happens because more surface area of the piece of magnesium will be exposed to the collisions of the other reactants. One way to think about this is that you have a block of magnesium or flakes of magnesium both with the same mass. The big block of magnesium has a lot of magnesium that you cannot see or touch because it is in the center of the block (it has a low surface area to mass ratio). But the flakes allow you to see most of the magnesium. The hydrochloric acid "sees" the magnesium and reacts with it so if more magnesium is readily available at the start of the reaction so it will happen faster.
(picture from http://www.brooklyn.cuny.edu/bc/ahp/..._ProbSize.html)
If you want more information about factors that affect reaction rates click here.
Q12.5.6
How does an increase in temperature affect rate of reaction? Explain this effect in terms of the collision theory of the reaction rate.
Answer 12.5.6:
To answer this question we first have to define collision theory. Collision theory states that for a reaction to take place all the needed reactants have to collide with one another. Collision theory is often times used to predict the rate of a reaction. It is important to note that not all collisions cause a chemical change. For a chemical change to take place there has to be a minimum amount of internal energy. This is also known as activation energy. The reacting species also have to be oriented in a manner that will allow them to collide correctly and react. So the rate of a reaction is related to the frequency of reactants colliding in the proper conditions. If you are still confused about collision theory click here.
An increase in temperature will speed up the rate of reactions by putting more energy into the system. Molecules will move faster and collide more. The more collisions there are the more likely it is that the molecules will collide correctly and cause a reaction.
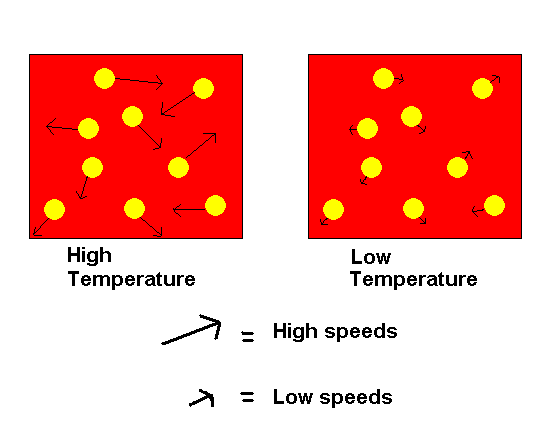
Q21.4.1
What are the types of radiation emitted by the nuclei of radioactive elements?
Answer 21.4.1:
| Type: | Symbol | Atomic # of element | Mass # of element | Charge of particle | Equation Example |
| Alpha (α) Decay | \(^{4}_{2}\textrm{He}\) | decreases by 2 | decreases by 4 | +2 | \[^{234}_{91}\textrm{Pa}\rightarrow^{230}_{89}\textrm{Ac} +^{4}_{2}\alpha\] |
| Beta (β-) Decay (electron) | \(^{0}_{-1}\textrm{β}\) | decreases by 1 | stays the same | -1 | \[^{14}_{6}\textrm{C}\rightarrow^{14}_{7}\textrm{N} + ^{0}_{-1}\beta\] |
| Beta (β+) Decay (positron) | \(^{0}_{-1}\textrm{β}\) | decreases by 1 | stays the same | +1 |
\[^{14}_{6}\textrm{C}\rightarrow^{14}_{5}\textrm{B} + ^{0}_{+1}\beta^+\] |
| Neutron Emission | \(^{1}_{0}\textrm{n}\) | stays the same | decreases by 1 | 0 | \[^{13}_{4}\textrm{Be}\rightarrow^{12}_{4}\textrm{Be} + ^{0}_{1}\textrm{n}\] |
| Gamma Decay (ray) | \(^{0}_{0}\textrm{γ}\) | stays the same | stays the same | 0 | \[^{234}_{91}\textrm{Pa*}\rightarrow^{234}_{91}\textrm{Pt} + ^{0}_{0}\gamma\] |
| Electron Capture | \(^{0}_{-1}\textrm{e}\) | decreases by 1 | stays the same | -1 |
\[^{56}_{27}\textrm{Co} + ^{0}_{-1}\textrm{e}\rightarrow^{56}_{26}\textrm{Fe} + \textrm{x-ray}\] |
\(^{A}_{B}\textrm{X}\)
A: Mass of the atom
B: Number of protons in the atom
X: The atom
This is just a basic outline of what each type of radiation does not why it happens. If you would like more information click here
Q20.2.5
Of the following elements, which would you expect to have the greatest tendency to be oxidized: Zn, Li, or S? Explain your reasoning.
Answer 20.2.5:
There are two ways to solve this problem:
When looking at this problem it is first important to know what oxidation is. Oxidation is the loss of electrons. If an element has a high tendency to be oxidized it means that the element easily gives up its electrons. This is related to electronegativity, a very electronegative element does not give its electrons up easily while a weak electronegative element does. The electronegativity trend decreases down a group and increases across a period.
The first way is to find the element that has the greatest tendency to be oxidized you have to find the least electronegative element by finding Zn, Li, and S on the periodic table. When you do that you will see that Li is the least electronegative element of the three and is more likely to be oxidized.
The second way to solve this problem is to look at the reduction potential of each element. Reduction potential measures the ability of a chemical species to be reduced (gain electrons). By looking at the reduction potential of each element on the activity series here you will see that Li has the lowest reduction potential of Zn, Li and S making it the element that has the greatest tendency to be oxidized.
The third way is to test these elements experimentally. In order to see which element has the highest tenancy to be oxidized, we can use these elements as a sort of sacrificial anodes. By placing these elements within a solution where they can be oxidized in an oxidizing acidic solution. The element with the most amount of corrosion will support that it has the highest tenancy to oxidize since oxidation within metals also correlate the corrosion of the metal as well.
Q20.4.24
Your lab partner wants to recover solid silver from silver chloride by using a 1.0 M solution of HCl and 1 atm H2 under standard conditions. Will this plan work?
Answer 20.4.24:
To solve this problem we first have to find the overall equation:
\[{2}\textrm{AgCl} +\textrm{H}_2\rightarrow{2}\textrm{HCl} + {2}\textrm{Ag}\]
From this reaction we know that H2 has to "kick out" silver from silver chloride for this reaction to work. We can see if H2 will kick out silver by looking at standard reduction potentials:
\[\textrm{Ag}^+ +\textrm{e}^-\rightarrow\textrm{Ag}\] Eo=0.80V
\[{2}\textrm{H}^+ + {2}\textrm{e}^-\rightarrow\textrm{H}_2\] Eo= 0.00V
Now we have to assign the oxidation and reduction states for each reaction:
We do this by looking at the overall reaction:
Ag gains an electron and H loses an electron. Because of this the silver reaction is reduction and the H2 reaction is oxidation.
Using this information we can use:
Eocell = Eocathode - Eoanode,
Eocell = 0.800V - 0V
Eocell = +0.800V
Because Eocell is positive the reaction will be spontaneous so the lab partner's method of recovering solid silver will work.
For more about cell potentials visit (https://chem.libretexts.org/Textbook...thermodynamics )
Q20.9.9
What mass of PbO2 is reduced when a current of 5.0 A is withdrawn over a period of 2.0 h from a lead storage battery?
Answer 20.9.9:
Because this is a battery there is a redox equation going on inside. By looking at the standard reduction potential table we get:
\[Pb^2+ +2e^- \rightarrow Pb(s) \]
We look at the lead part of the reaction because it is being reduced in PbO2.
To solve this problem we need to use this equation:
\[\textrm{n}=\dfrac{\textrm{I x t}}{\textrm{F}}\]
I= current (amps), t= time (seconds), F= Faraday’s constant (9.65x104 C/mole), n= # of moles of e-'s
An amp is a rate of Columbus per second.
This equation allows us to convert current to moles of electrons from there we can convert moles of electrons to mass.
By looking at the problem we see that I=5.0 A, T=2.0 h and F= 9.65x104 C/mole we are trying to find n.
We first have to convert time into seconds from hours we do this by converting hours to minutes then minutes into seconds:
\[ 2\text{ hours}\times\frac{60\text { minutes}}{1\text{ hour}}\times\frac{60\text { seconds}}{1\text{ minute}} = 7200\text{ seconds}\]
Next we "plug and chug" by plugging in all the known numbers into the equation:
\[ \text{ n}\ =\frac{5\text { A}\times 7200\text { seconds}}{96500 \text{ C/mole}}\]
\[ \text{ n}\ = .373 \text{ moles of electrons}\]
Because 2 electrons are transferred in the equation above we have to divide .373 by 2.
\[\dfrac{.373\text{ moles of electrons}}{2\text{ electrons transferred}} = .1865\text{ moles}\]
Now that we have the moles of electrons we can convert from moles to grams.
We do this by multiplying the number of moles by the mass of PbO2.
\[.1865\text{ moles}\times\dfrac{239.2\text{ grams}}{1\text{ mole}} = 44.61\text{ grams}\]
44.61 grams of PbO2 is reduced when 5.0 A is withdrawn over a period of 2.0 h from a lead storage battery.
Q14.6.7
Above approximately 500 K, the reaction between NO2 and CO to produce CO2 and NO follows the second-order rate law Δ[CO2]/Δt = k[NO2][CO]. At lower temperatures, however, the rate law is Δ[CO2]/Δt = k′[NO2]2, for which it is known that NO3 is an intermediate in the mechanism. Propose a complete low-temperature mechanism for the reaction based on this rate law. Which step is the slowest?
Answer 14.6.7:
Because we know that NO3 is an intermediate we know that it won't show up in the final reaction and will be a product of one reaction and a reactant in the next.
First we need to write out the overall reaction: \[CO(g) + NO_2(g) \rightarrow CO_2(g) + NO(g)\]
Knowing what we start with we can come up with 2 equations with an intermediate that when added together will equal the overall reaction
\[2NO_2(g) \rightarrow NO_3(g) + NO(g) \]
\[CO(g) + NO_3(g) \rightarrow CO_2(g) + NO_2(g) \]
The rate of a reaction depends on the concentrations of the reactants. By looking at the equations you can tell that the first equation has NO2 as a reactant just like the overall rate does.
When you set it up you get: \[\textrm{k}^′\textrm{[NO}_2\textrm{]}^2\]
This means that the first reaction is the slow step.
If you weren't sure what step was the slow step you can guess and check. But it is first important to see if either of the steps obviously work with the given rate.
For more about rate law you can check out (https://chem.libretexts.org/Core/Phy...tics/Rate_Laws).

