Extra Credit 18
- Page ID
- 82723
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Q17.2.7
Why is a salt bridge necessary in galvanic cells like the one below?

Solution:
The salt bridge is necessary in galvanic cells to maintain electrical neutrality within the internal circuit, keeping the redox reaction neutral. The purpose of a salt bridge is to maintain charge balance because the electrons are moving from the anode (oxidation) half cell to the cathode (reduction) half cell. In other words, for a galvanic cell to be useful, it must remain out of equilibrium and by adding the salt bridge. With a salt bridge, each half-cell remains electrically neutral and current can continue to flow through the circuit, keeping the cell out of equilibrium. A salt bridge consists of an ionic compound in is aqueous solution form that moves across cells to maintain the overall neutrality of the cell.
Q19.1.16
Predict the products of each of the following reactions.
a. \(MnCO_{3}(s)+HI(aq)\rightarrow\)
b. \(CoO(s)+O_{2}(g)\rightarrow\)
c. \(La(s)+O_{2}(g)\rightarrow\)
d. \(V(s)+VCl_{4}(s)\rightarrow\)
e. \(Co(s)+xsF_{2}(g)\rightarrow\)
f. \(CrO_{3}(s)+CsOH(aq)\rightarrow\)
Solution:
To know if the product will result in a precipitate, there are solubility rules:
- Salts formed with group 1 cations and \(NH_{4}^{+}\) cations are soluble. There are some exceptions for certain \(Li^{+}\) salts.
- Acetates (\(C_{2}H_{3}O_{2}^{-}\)), nitrates (), and perchlorates (\(ClO_{4}^{-}\)) are soluble.
- Bromides, chlorides, and iodides are soluble.
- Sulfates () are soluble with the exception of sulfates formed with
- Salts containing silver, lead, and mercury (I) are insoluble.
- Carbonates (\(CO_{3}^{2-}\)), phosphates (\(PO_{4}^{3-}\)), sulfides, oxides, and hydroxides () are insoluble. Sulfides formed with group 2 cations and hydroxides formed with calcium, strontium, and barium are exceptions.
- If the rules state that an ion is soluble, then it remains in its aqueous ion form. If an ion is insoluble based on the solubility rules, then it forms a solid with an ion from the other reactant. If all the ions in a reaction are shown to be soluble, then no precipitation reaction occurs.
- For net ionic equations, first determine the type of reaction it will be.
- Recall:
- The equation for the double replacement reaction: AB(aq) + CD(aq) → AD(aq) + CB(s)
- The equation for synthesis reaction: A + B → AB
- Recall:
- Second, consult the solubility rules to determine if the products are soluble.
- Third, determine if the products are stable or unstable, and break down the unstable products into stable products.
- Lastly, balance the equation.
If all products are aqueous, a net ionic equation cannot be written because all ions are canceled out as spectator ions. Therefore, no precipitation reaction occurs.
a) First do the equation for the double replacement \[MnCO_{3}(s)+2HI(aq)\rightarrow H_{2}CO_{3}(s)+MnI_{2}(aq)\]
Since \(H_{2}CO_{3}(s)\) is unstable, it will breakdown into: \(H_{2}CO_{3}(s) \to H_2O(l) + CO_2(g)\)
Therefore, the final equation would be: \(MnCO_{3}(s)+2HI(aq)\rightarrow H_2O(l) + CO_2(g) + MnI_2(s)\)
b) This is a synthesis reaction
\[CoO(s)+O_{2}(g)\rightarrow Co_2O_3(s)\] note: The change in charge.
Balance the equation and the final answer would be:
\[4CoO(s)+ O_{2}(g)\rightarrow 2Co_2O_3(s)\]
c) This is like the synthesis reaction above
\[4La(s)+3O_{2}(g)\rightarrow 2La_{2}O_{3}(s)\]
d) Combine both the solids and then balance the equation. \[V(s)+3VCl_{4}(s)\rightarrow 4VCl_{3}(s)\]
e) Cobalt can be in oxidation states +2 and +3 so the reaction states can be \[Co(s)+F_{2}(g)\rightarrow CoF_{2}(g)\]
\[2Co(s)+3F_{2}(g)\rightarrow 2CoF_{3}(g)\]
The symbol "xs" indicates that F2 is added in excess so the second reaction is favored more as it drives the reaction to completion.
\[2Co(s)+xsF_{2}(g)\rightarrow 2CoF_{3}(g)\]
f) The hydroxide anion can be used to become a water molecule as a product. After that, you can balance the rest of the reaction. \[CrO_{3}(s)+2CsOH(aq)\rightarrow CsCrO_{4}(s)+H_{2}O(l)\]
Note: Most of the original solution is correct, but some parts were incorrect.
Q19.3.8
For complexes of the same metal ion with no change in oxidation number, the stability increases as the number of electrons in the t2g orbitals increases. Which complex in each of the following pairs of complexes is more stable?
a. \([Fe(H_{2}O)_{6}]^{2+}\) or \([Fe(CN)_{6}]^{4-}\)
b. \([Co(NH_{3})_{6}]^{3+}\) or \([CoF_{6}]^{3-}\)
c.\([Mn(CN)_{6}]^{4-}\) or \(MnCl_{6}]^{4-}\)
Solution:
The Spectrochemical Series is as follows \[I^{-}<Br^{-}<SCN^{-}\approx Cl^{-}<F^{-}<OH^{-}<ONO^{-}<ox<H_{2}O<SCN^{-}<EDTA<NH_{3}<en<NO_{2}^{-}<CN\]
The strong field ligands (on the right) are low spin which fills in more electrons in the t2g orbitals. The weak field ligands (on the left) are high spin so it can fill electrons in the t2g orbitals and eg orbitals. In conclusion, more electrons are filled up from the strong field ligands because the electrons don't move up to the eg orbitals.
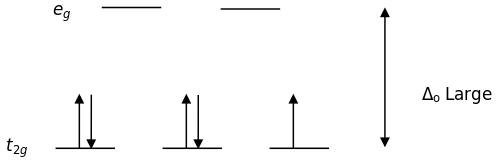
a. \([Fe(CN)_{6}]^{4-}\)
\(CN\) is a stronger ligand than \(H_{2}O\) so it is low spin, which fills up the t2g orbitals.
b. \([Co(NH_{3})_{6}]^{3+}\)
\(NH_{3}\) is a strong ligand than \(F\).
c. \([Mn(CN)_{6}]^{4-}\)
\(CN\) is a stronger ligand than \(Cl^{-}\).
For more information regarding the shape of the complex and d-electron configuration, libretext provides more information on how to classify high and low spin complexes.
Q12.4.8
What is the half-life for the decomposition of NOCl when the concentration of NOCl is 0.15 M? The rate constant for this second-order reaction is 8.0 × 10−8 L/mol/s.
Solution:
The half-life equation for second-order reactions is \[t_{\frac{1}{2}}=\frac{1}{k[A]_{0}}\]
From the question above, we know that \(k=8.0*10^{-8} L/mol/s\) and \([A]_{0}=0.15M\).
By plugging in the two variables, we get \[t_{\frac{1}{2}}=\frac{1}{(8.0*10^{-8} L/mol/s)(0.15M)}\]
which gives us the half-life of NOCl equal to 8.3 × 107 s.
Q21.2.3
For the following isotopes that have missing information, fill in the missing information to complete the notation
a. \(_{14}^{34}\textrm{X}\)
b. \(_{X}^{36}\textrm{P}\)
c. \(_{X}^{57}\textrm{Mn}\)
d. \(_{56}^{121}\textrm{X}\)
Solution:
The notation for elements is \(_{Z}^{A}\textrm{Element Symbol}\) where \({Z}\) is the atomic number and \({A}\) is the mass number. The easiest method to fill in the missing information is to look at the number of protons \({(Z)}\) and locate the element from the periodic table. From there, you can find the missing atomic numbers as well as the missing element symbols.
a. \(_{14}^{34}\textrm{Si}\)
Look for the atomic number 14 on the periodic table because that corresponds to its element symbol.
b. \(_{15}^{36}\textrm{P}\)
Look for "Phosphorus" on the periodic table to determine its atomic number.
c. \(_{25}^{57}\textrm{Mn}\)
Look for "Manganese" on the periodic table to determine its atomic number.
d. \(_{56}^{121}\textrm{Ba}\)
Look for the atomic number 56 on the periodic table because that corresponds to its element symbol.
Q21.5.6
In usual practice, both a moderator and control rods are necessary to operate a nuclear chain reaction safely for the purpose of energy production. Cite the function of each and explain why both are necessary.
Solution:
The function of a moderator in a nuclear chain reaction is to reduce the speed of fast neutrons produced from nuclear fission. The moderator is necessary because by slowing the neutrons down the probability of a neutron interacting with Uranium-235 nuclei is greatly increased. This helps maintain the chain reaction. The control rods for a reactor are used to stick down into the fuel to absorb neutrons, to slow the reaction down, or withdraw to speed the reaction up. The control rod is necessary to control the reactor and keep it in steady state.
Q20.4.4
If the components of a galvanic cell include aluminum and bromine, what is the predicted direction of electron flow? Why?
Solution:
The predicted direction of electron flow would be from aluminum (the anode where oxidation occurs) to bromine (the cathode where reduction occurs). The relative equation could be \[Al(s)+Br_{2}(l)\rightarrow Al^{3+}(aq)+2Br^{-}(aq)\]
The half reactions for aluminum and bromine are \[Al(s)\rightarrow Al(s)Al^{3+}(aq)+3e^{-}\]
\[Br_{2}(l)+2e^{-}\rightarrow 2Br^{-}(aq)\]
Electrons in a galvanic cell flow from the anode to the cathode. Aluminum is being oxidized because it goes from a charge of 0 to +3. Bromine is being reduced because it goes from a charge of 0 to -2. Oxidized means to lose electrons and reduced means to gain electrons.
Additionally, by looking at the reduction potentials, we can determine if a reaction is going to happen at the anode or cathode by looking at the \(E^o\) for each half reaction.

Based on the chart:
\[Al(s)\rightarrow Al(s)Al^{3+}(aq)+3e^{-} \quad E^o = -1.66 V \]
\[Br_{2}(l)+2e^{-}\rightarrow 2Br^{-}(aq) \quad E^o = 1.09 V\]
The half reaction the produces the lowest voltage compared to the other half reaction is the reaction at the anode, and will be the oxidation reaction.
Q20.7.2
Why does the density of the fluid in lead–acid batteries drop when the battery is discharged?
Solution:
A lead-acid battery consists of two lead plates, a positive plate covered with lead dioxide and a negative plate made of lead. The density of the fluid in lead-acid batteries drop when the battery is discharged because when electricity flows through the electrolytes and water, \(H_{2}O\) is converted into its original elements, hydrogen and oxygen. This causes water loss and therefore lead acid batteries need to have water added periodically to keep the density to its original content.
(s)+CsOH(aq)⟶
((
(s)+CsOH(aq)⟶

