8.13: Organic Compounds-Some Additional Classes
- Page ID
- 49460
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Although a tremendous number of different hydrocarbons occur as a result of carbon’s ability to bond in long chains, an even greater variety of substances is possible when oxygen, nitrogen, and several other elements with carbon and hydrogen. The presence of highly electronegative atoms like oxygen or nitrogen in combination with hydrogen permits hydrogen bonds to form between molecules of many of these substances. We have deferred discussion of their properties until now so that you can apply your knowledge of hydrogen bonding to them.
Elsewhere, we mentioned that alkanes were relatively unreactive and that the presence of a double or triple bond made unsaturated molecules more likely to combine chemically. A site which makes an organic molecule more reactive than a simple hydrocarbon chain is called a functional group. Many of the important organic functional groups involve oxygen atoms, nitrogen atoms, or both. We will discuss substances containing some of these functional groups in this section.
Organic Functional Groups
In addition to having different shapes, organic molecules can combine carbon backbones with other atoms, such as oxygen, nitrogen, or sulfur, to create functional groups. The specific arrangement of these atoms cause molecules to have certain properties. For instance, carboxylic acids always end up having a low pH. Chemists have long studied different compounds in nature, and many of the names for functional groups came about before we even understood what combination of atoms created their function. In organic chemistry, putting a dash before something like “-OH” means the O is bonded to the carbon structure. An R or R’ is used to denote “more of the carbon structure goes here.” See the example pictures. In the following pages of boxes.
|
Functional Group |
Identified by: |
Suffix |
|---|---|---|
|
-OH attached to a carbon with only carbons and hydrogens |
-ol |
|
|
-NH2, -NHR, or -NR2 attached to a carbon with only carbons and hydrogens otherwise attached to it. |
-amine |
|
|
Carbonyl |
=O attached to a carbon. Carbonyls give a large range of functionality, so they are broken down into the following: |
|
|
=O attached to a carbon on the end of the chain. |
-al |
|
|
=O attached to a carbon in the middle of the chain. |
-one |
|
|
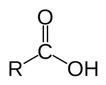
=O attached to a carbon that also has an alcohol: -OH. Denoted: -COOH, -CO2H, -C(O)OH, or:
|
-oic acid (when acidic) -ate (when basic) |
|
|
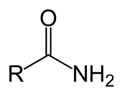
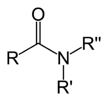
=O attached to a carbon that also has an amine, -NH2, -NHR, or -NR2 attached to it.
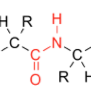
The central structure: with one H on the N, and a carbon chain continuing on both ends is commonly called a “peptide link” or “peptide bond” |
-amide or peptide |
|
|
Thiol |
-S-H attached to a carbon at the end of the chain |
-thiol |
|
Sulfide Disulfide |
-S- in the middle of the chain -S-S- in the middle of the chain |
-sulfide -disulfide |