8.14: Alcohols
- Page ID
- 49462
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Molecules of alcohols contain one or more hydroxyl groups (OH groups) substituted for hydrogen atoms along the carbon chain. The structure of the simplest alcohol, methanol (methyl alcohol), can be derived from that of methane by putting an OH in place of one of the H’s:
| Methane | Methanol |
|---|---|
The name, too, is derived from the name methane by replacing the final e with ol (for alcohol). The general formula for an alcohol may be written as R—OH, where R represents the hydrocarbon (alkane) portion of the molecule and is called an alkyl group. In methanol, R is the methyl group CH3.
Methanol is also called wood alcohol because it can be obtained by heating wood in the absence of air, a process called destructive distillation. Methanol vapor given off when the wood is heated can be condensed to a liquid by cooling below its boiling point of 65°C. The effect of polarity and especially hydrogen bonding due to the OH group is evident when this is compared with the temperature of –85°C at which ethane, C2H6, boils. Both molecules contain 18 electrons and are nearly the same size, and so London forces should be about the same, but the OH group in one methanol molecule can form strong hydrogen bonds with an OH in another molecule. Methanol is an important industrial chemical—nearly 3 × 1010 kg was produced worldwide in 2003[1]. Some was made by destructive distillation, but most was synthesized from hydrogen and carbon monoxide:
\[\ce{2H_{2} (g) + CO (g) \longrightarrow CH_{3}OH(l)} \nonumber \]
This reaction is carried out at pressures several hundred times normal atmospheric pressure, using metal oxides as catalysts. Methanol is mainly used to make other compounds from which plastics are manufactured, but some is consumed as fuel in jet engines and racing cars. Methanol is also a component of nonpermanent antifreeze and automobile windshield-washer solvent.
The second member of the alcohol family is ethanol (ethyl alcohol)― the substance we commonly call alcohol. Ethanol is also known as grain alcohol because it is obtained when grain or sugar ferments. Fermentation refers to a chemical reaction which is speeded up by enzymes and occurs in the absence of air.
Ethanol can also be synthesized by adding H2O to ethene, obtained during petroleum refining:
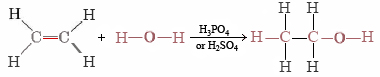
This is a typical example of an addition reaction. The H and OH from H2O are added to the ethene molecule and held there by electrons made available when one-half of the double bond breaks.
Ethanol is used as a solvent, in some special fuels, in antifreeze, and to manufacture a number of other chemicals. You are probably most familiar with it as a component of alcoholic beverages. Ethanol makes up 3 to 6 percent of beer, 12 to 15 percent of most wines, and 49 to 59 percent of distilled liquor. (The “proof” of an alcoholic beverage is just twice the percentage of ethanol.) Alcohol’s intoxicating effects are well known, and it is a mild depressant. Prolonged overuse can lead to liver damage. Methanol also produces intoxication but is much more poisonous than ethanol—it can cause blindness and death. Denatured alcohol is ethanol to which methanol or some other poison has been added, making it unfit for human consumption. Most of the ethanol not used in alcoholic beverages is denatured because in that form its sale is taxed at a much lower rate.
Two isomers are possible for alcohols containing three carbon atoms:
| The two structural isomers of propanol, 1-propanol and 2-propanol(isopropyl alcohol) |
The 1 and the 2 in the names of these compounds indicate the position of the OH group along the carbon chain. The propanols are much less important commercially than methanol and ethanol, although 2-propanol is commonly found in rubbing alcohol.
A carbon atom typically forms four bonds. Therefore, in an alcohol where carbon is bonded to an -OH group, there can be up to three carbon atoms directly bonded to the carbon atom bonded to the oxygen in -OH. If no carbon atom or one carbon atom is bonded directly, the compound is a primary alcohol. If two are bonded directly, it is a secondary alcohol; with three it is a tertiary alcohol, as illustrated below.
| These three molecule are representative of a primary alcohol (1-butanol), secondary alcohol (2-butanol) and a tertiary alcohol (2-methyl-2-propanol). |
All alcohols can be completely oxidized to carbon dioxide and water by oxygen in the air; that is, all alcohols are combustible. Like hydrocarbons, combustion is an important reaction of alcohols, but more controlled oxidation is even more important, because it can convert alcohols into other compounds that are useful to society. The ease with which an alcohol can be oxidized and the extent of the oxidation depends on whether the alcohol is primary, secondary, or tertiary.
For primary alcohols, controlled, stepwise oxidation first yields compounds called aldehydes; if more of the oxidizing agent is available, then aldehydes can be further oxidized to carboxylic acids. Schematically
Primary alcohol → aldehyde → carboxylic acid
Oxidation of an organic compound can usually be recognized because either an oxygen atom is added to a molecule or two hydrogen atoms are lost from a molecule. For example, stepwise oxidation of ethanol first produces the aldehyde ethanal (commonly called acetaldehyde); further oxidation produces the carboxylic acid, ethanoic acid (commonly called acetic acid). An aldehyde has the functional group –CHO, where the carbon atom is double-bonded to an oxygen atom. A carboxylic acid has the functional group –COOH, in which the carbon atom is double-bonded to an oxygen atom and single-bonded to an oxygen atom in an OH group. The structures below show the differences between ethanol, ethanal, and ethanoic acid.
| when oxidized gives and further oxidation gives |
Note that acetaldehyde differs from ethanol by loss of one H atom from the oxygen atom and one H atom from the carbon on the right. Acetic acid differs from acetaldehyde by having an addition O atom on the right-hand carbon atom.
Controlled oxidation can be carried out in the laboratory using an aqueous solution of potassium permanganate or an aqueous solution of potassium dichromate. When a similar controlled oxidation is applied to a secondary alcohol, such as 2-propanol (see below), the oxidized molecule contains a C=O group that has two other carbon atoms attached to the C atom. This >C=O group with two carbon atoms attached to the C is called a ketone.
Again, note that in the ketone the number of hydrogen atoms is fewer by two than the number in the secondary alcohol. The oxidation corresponds with loss of two hydrogen atoms. Ketones are difficult to oxidize further, because there is no way to add another oxygen atom to the carbon atom in the >C=O group, nor is there a way to remove hydrogen atoms from the C and O atoms in the >C=O group.
Tertiary alcohols, which have no hydrogen atoms attached to the carbon that is bonded to the –OH group, are difficult to oxidize. If a primary alcohol, a secondary alcohol, and a tertiary alcohol are dissolved in water in three beakers and then treated with either potassium permanganate or potassium dichromate, only the primary and secondary alcohols will react. (The reaction can be observed because both permanganate ions and dichromate ions are colored (purple and orange, respectively). Thus for the primary and secondary alcohols, the color will disappear, but for tertiary alcohols there will be no color change.
The structures of two more alcohols which are of commercial importance and are familiar to many persons are shown below:
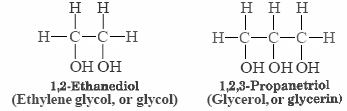
Each ethylene glycol molecule has two hydrogens which can participate in hydrogen bonding, and each glycerin molecule has three. Both substances have rather high boiling points (198°C for ethylene glycol and 290°C for glycerin) and are syrupy, viscous liquids at room temperature. Their resistance to flowing freely is due to the network of hydrogen bonds that links each molecule to several of its fellows, making it more difficult for them to slide past one another. This highlight again the effect of hydrogen bonding on intermolecular forces and physical properties. In fact, in our table of the boiling points of comparable organic compounds ethylene glycol has the highest boiling point compared with other compounds containing the same number of electrons. The two propanol isomers are also on this table, only exceeded in boiling point by ethylene glycol, and acetic acid.
Ethylene glycol is the principal component of engine coolant for automobiles and is also used to manufacture polyester fibers. In 2005, nearly 1.8 × 1010 kg was produced worldwide. [2] Glycerin is used as a lubricant and in the manufacture of explosives:
When nitroglycerin is mixed with a solid material such as nitrocellulose (which is made by treating cotton or wood pulp with nitric acid), the product is a form of dynamite.
References
- Pritchard, J.D. "Methanol - Production and Uses." Health Protection Agency. 22 October 2008. www.hpa.org.uk/web/HPAweb&.../1195733803581
- Pritchard, J.D. "Ethylene Glycol - Production and Uses." Health Protection Agency. 15 October 2008. www.hpa.org.uk/webw/HPAweb&am...=1190384322220


