8.17: Carboxylic Acids
- Page ID
- 49467
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Serving wine usually involves a rather elaborate ceremony in which the host tastes the wine before pouring it for the guests. One reason for this is the possibility that the wine may have been spoiled by exposure to air.
Certain bacterial enzymes are capable of converting ethanol to ethanoic acid (acetic acid) when oxygen is present:
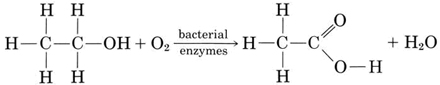
The same reaction occurs when cider changes into vinegar, which contains 4 to 5 percent acetic acid. Acetic acid gives vinegar its sour taste and pungent odor and can do the same thing to wine.
Acetic acid, CH3COOH, is an example of the class of compounds called carboxylic acids, each of which contains one or more carboxyl groups, COOH. The general formula of a carboxylic acid is RCOOH. Some other examples are
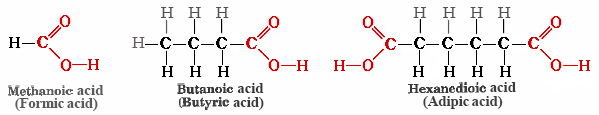
Formic acid (the name comes from Latin word formica meaning “ant“) is present in ants and bees and is responsible for the burning pain of their bites and stings. Butyric acid, a component of rancid butter and Limburger cheese, has a vile odor. Adipic acid is an example of a dicarboxylic acid—it has two functional groups—and is used to make nylon.
Since the carboxyl group contains a highly polar 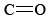 as well as an OH group, hydrogen bonding is extensive among molecules of the carboxylic acids. Pure acetic acid is called glacial acetic acid because its melting point of 16.6°C is high enough that it can freeze in a cold laboratory. As you can see from the table of boiling points, acetic acid boils at a higher temperature than any other organic substance whose molecules are of comparable size and have but one functional group. It is also quite thick and syrupy because of extensive hydrogen bonding.
as well as an OH group, hydrogen bonding is extensive among molecules of the carboxylic acids. Pure acetic acid is called glacial acetic acid because its melting point of 16.6°C is high enough that it can freeze in a cold laboratory. As you can see from the table of boiling points, acetic acid boils at a higher temperature than any other organic substance whose molecules are of comparable size and have but one functional group. It is also quite thick and syrupy because of extensive hydrogen bonding.
Below is a Jmol model of acetic acid. In the general menu to the left, click on partial charges. Each atom in the molecule will be assigned a partial charge. It is clear that the oxygen atoms are sharing electrons unequally and causing other parts of the molecule to gain a partial positive charge in the carboxyl carbon and hydrogen. Further, this induces a partial negative charge on the methyl carbon, leading to positive charges on the methyl hydrogen atoms.
An even better way to view the electron distribution is with the Molecular Electrostatic Potential (MEP) Surface options. One can look at "MEP on isopotential surface", which show surfaces where electrostatic potential is the same, but the most informative option here is the "MEP on Van der Waals Surface" radio button. This shows the potential along the van der Waals surface of the molecule. The closer to red on the color spectrum, the more negative the potential at that surface is, the closer to blue, the more positive. One can see that both oxygen atoms are centers of partial negative charge, while the acidic hydrogen atom has a substantial partial positive charge, and the methyl group is also has a partial positive charge. One more way to look at the molecule, is to use the "MEP on a plane" button. Choose the XY plane, and then click "Set Plane Equation." This will show the electrostatic potential along the axis of symmetry for the molecule. While two hydrogen atoms on the methyl group are out of the plane, this view still allows one to see how partial charge is distributed along the backbone of the molecule in a way the van der Waals surface does not. From this modeling of the acetic acid molecule, hopefully it is becoming clear how the macroscopic properties we discussed arise.
Acetic acid is synthesized commercially according to the reaction shown above, but silver is used as a catalyst instead of bacterial enzymes. It is also prepared by reading air with propane separated from natural gas. The liquid acetaldehyde obtained in this reaction is then combined with oxygen in the presence of manganese(II) acetate to make acetic acid. About half the acetic acid produced in the United States goes into cellulose acetate from which acetate fibers are made.


