6.4: Effective Nuclear Charge and Shielding
- Page ID
- 465536
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Estimate Time to Read: 8 min
Coulomb's Law
Coulomb's Law is from classical physics (Equation 1); it tells us that particles with opposite electrostatic charges (q1, q2) are attracted to each other, and the larger the charge on either particle or the closer the distance (r) between them, the stronger the force of attraction (F). Coulombs' law works well for predicting the energy of an electron in a hydrogen atom because H has only one electron. It also works for hydrogen-like atoms: any nucleus with exactly one electron (a He+ ion, for example, has one electron). However, Coulomb's law is insufficient for predicting the energies of electrons in multi-electron atoms and ions.Coulomb's law explains why atomic size decreases as the charge on the nucleus increases, but it can't explain the nuances and variations in size as we go across the periodic table. Coulomb's Law also explains why electrons in different shells (n), at different distances from the nucleus, have different energies. But on its own, Coulomb's law doesn't quite explain why electron subshells within a shell (like 2s vs. 2p) would have different energies. To explain these things, we need to consider how both electron shielding and penetration result in variations in effective nuclear charge (Z*) that depend on shell and subshell.
Equation \(\PageIndex{1}\). Coulomb's Law
\[ F=k \dfrac{ q_1 q_2}{r^2} \nonumber \]
Effective Nuclear Charge (Z*)
For an atom or an ion with only a single electron, we can calculate the potential energy by considering only the electrostatic attraction between the positively charged nucleus and the negatively charged electron. However, electrons within a multi-electron atom interact with the nucleus and with all other electrons. Each electron in a multi-electron atom experiences both attraction to the nucleus and repulsion from the other electrons. The presence of multiple electrons decreases the nuclear attraction to some extent. Each electron in a multi-electron atom experiences a different magnitude of (and attraction to) the nuclear charge depending on what specific shell and subshell the electron occupies. The amount of positive nuclear charge experienced by any individual electron is the effective nuclear charge (Z*).

For example, in lithium (Li), none of the three electrons "feel" the full +3 charge from the nucleus (Figure \(\PageIndex{1}\)). Rather, each electron "feels" a Z* that is less than the actual Z and that depends on the electron's orbital. The actual nuclear charge in Li is \(Z=+3\); the 1s electrons experience a \(Z^* =+2.69\), and the 2s electron experiences a \(Z^* = +1.28\). In general, core electrons (or the electrons closest to the nucleus), "feel" a Z* that is close to, but less than, Z. On the other hand, outer valence electrons experience a Z* that is much less than Z.
- Core electrons: \(Z^* \lessapprox Z\)
- Valence electrons: \(Z^* \ll Z\)
If an electron is far from the nucleus, then at any given moment most of the other electrons will be between that electron and the nucleus. Hence the electrons will cancel a portion of the positive charge of the nucleus and thereby decrease the attractive interaction between it and the electron farther away. The farther an electron is from the nucleus, the more electrons between that electron and the nucleus, and the greater the actual nuclear charge Z is than the effective nuclear charge Z*. This effect is called electron shielding.
Shielding
Shielding is the reduction of true nuclear charge (Z) to the effective nuclear charge (Z*) by other electrons in a multi-electron atom or ion. Shielding occurs in all atoms and ions that have more than one electron. H is the only atom in which shielding does not occur.
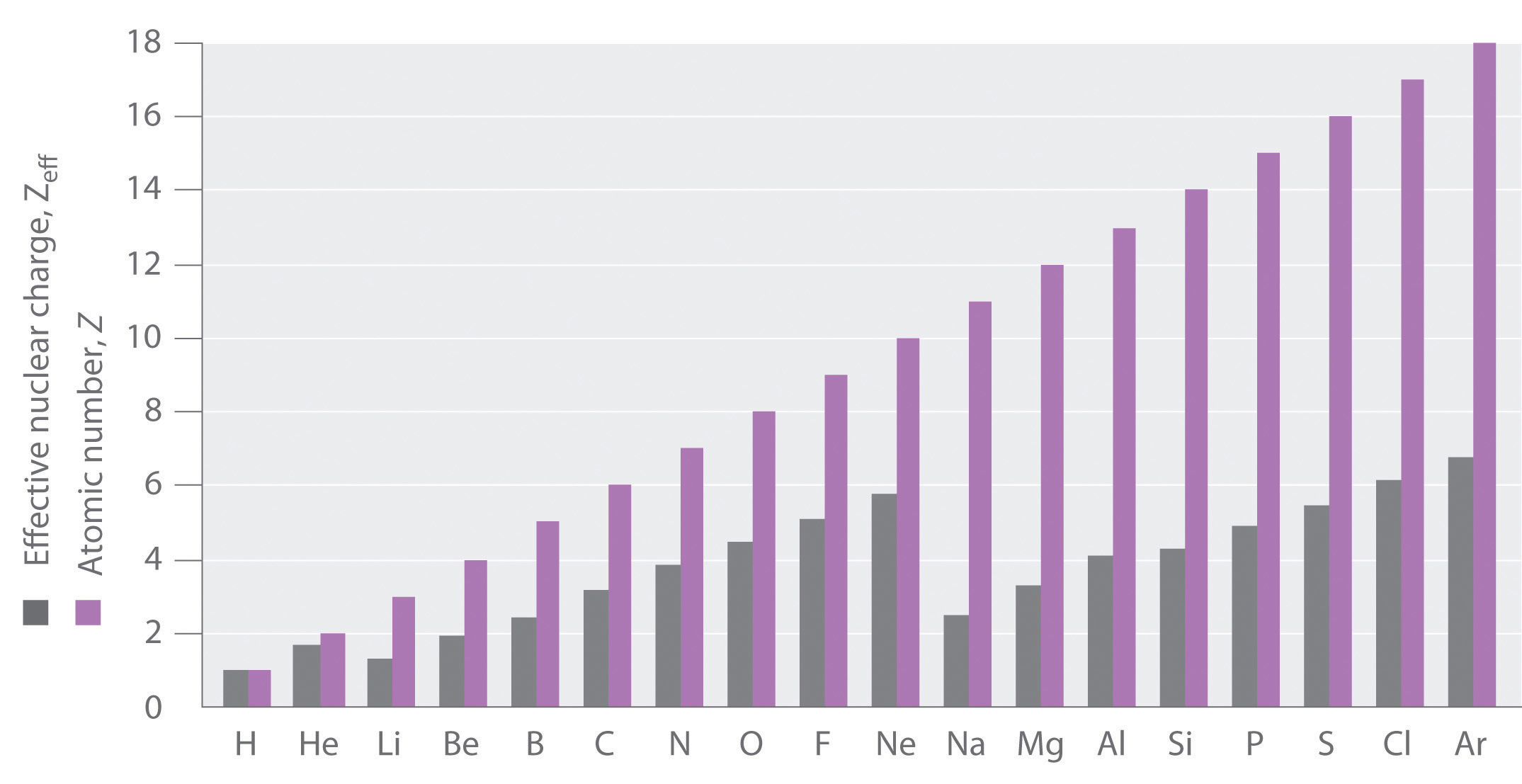
Electrons in a multi-electron atom interact with the nucleus and all other electrons in the atom. To describe shielding, we choose an electron-of-interest in a multi-electron atom and treat all the "other" electrons as a group. While the positive charge of the nucleus attracts our electron, the negative charges of the "other" electrons repel our electron-of-interest. The attractive and repulsive forces partially cancel each other; however, there are fewer "other" electrons than there are protons in our atom so the nuclear charge is never completely canceled. The "other" electrons partially block, or shield, part of the nuclear charge so that our electron-of-interest experiences a partially-reduced nuclear charge, the Z*.
In reality, there is not a one-for-one "canceling" of the nuclear charge by each electron. Partly due to penetration, no single electron can completely shield a full unit of positive charge. Core electrons shield valence electrons, but valence electrons have little effect on the Z* of core electrons. The ability to shield, and be shielded by, other electrons strongly depends the electron orbital's average distance from the nucleus and its penetration; thus shielding depends on both shell (\(n\)) and subshell (\(l\)).
Electron Penetration
Coulomb's law shows us that distance of an electron from its nucleus is important in determining the electron's energy (its attraction to the nucleus). The shell number (\(n\)) determines approximately how far an electron is from the nucleus on average. Thus, all orbitals in the same shell (s,p,d) have similar sizes and similar average distance of their electrons from the nucleus. But there is another distance-related factor that plays a critical role in determining orbital energy levels: penetration. Penetration describes the ability of an electron in a given subshell to penetrate into other shells and subshells to get close to the nucleus. Penetration is the extent to which an electron can approach the nucleus. Penetration depends on both the shell (\(n\)) and subshell (\(l\)).
The penetration of individual orbitals can be visualized using the radial probability functions, which show the probability of locating an electron in an orbital as a function of distance from the nucleus. Figure \(\PageIndex{3}\) shows the radial probability function of the 1s, 2s, and 2p orbitals. From these plots, we can see that the probability peak of the 1s orbital closest to the nucleus; thus it is the most penetrating. While the 2s and 2p have most of their probability at a farther distance from the nucleus (compared to 1s), the 2s orbital and the 2p orbital have different extents of penetration. Notice that the 2s orbital is able to penetrate the 1s orbital because of the central 2s lobe. The 2p orbital penetrates somewhat into the 1s, but it cannot approach the nucleus as closely as the 2s orbital can. While the 2s orbital penetrates more than 2p, the average probability of the 2p is slightly closer than that of the 2s. The order of Z* in 2s and 2p subshells depends on which factor (average distance or penetration) is more important. In the first two rows of the periodic table, penetration is the dominant factor that results in 2s having a lower energy than 2p.
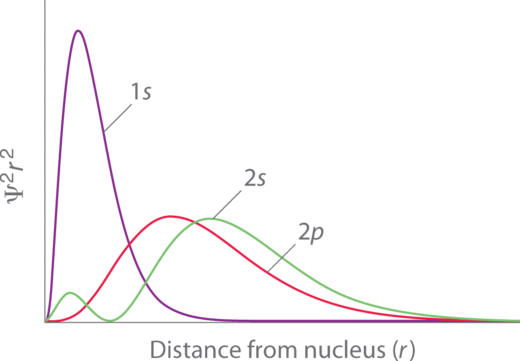
An electron orbital's penetration affects its ability to shield other electrons and affects the extent to which it is shielded by other electrons. In general, electron orbitals that have greater penetration experience stronger attraction to the nucleus and less shielding by other electrons; these electrons thus experience a larger Z*. Electrons in orbitals that have greater penetration also shield other electrons to a greater extent.
Within the same shell value (n), the penetrating power of an electron follows this trend in subshells (ml):
s > p > d > f
Problems
Compare the 2s and 2p orbitals:
- Which is closer to the nucleus on average?
- Which is more penetrating?
- Which orbital experiences a stronger Z* and is thus lower in energy. Explain.
- Answer
-
- The 2s orbital is closer to the nucleus on average.
- The 2s orbital is more penetrating than 2p.
- You might "know" that the 2s orbital is lower in energy than 2p because 2s fills first. But a close inspection of Figures 1.1.2.3 and 1.1.2.4 indicates that while the 2s and 2p elements are degenerate in Ne (element 10), for elements with atomic number 11 and greater 2p has a higher Z* than 2s! This example illustrates that both average distance and penetration are factors in determining Z*, and the factor that is more important may change as we increase in atomic number.
Peruse the Hyperphysics page that shows radial probability functions of several orbitals (click around on various orbitals). Compare the 2p and 3s orbitals:
- Which is farther from the nucleus on average?
- Which is more penetrating?
- Which orbital is lower in energy?
- Answer
-
- The 3s orbital reaches farther away from the nucleus and is on average farther from the nucleus than 2p.
- The 3s orbital is more penetrating than 2p, even though 3s is farther on average!
- The 2p orbital is lower in energy than 3s; this is because 2p is still significantly closer to the nucleus on average and experiences a stronger Z*. (Penetration is not the only consideration!)
Which atom, Li, or N, has a stronger valence Z*? Explain why.
- Answer
-
A nitrogen atom has a stronger effective nuclear charge (Z*) than lithium due to its greater number of protons; even though N also has more electrons that would shield the nuclear charge, each electron only partially shields each proton. This means that atoms with greater atomic number always have greater Z* for any given electron.
Explain why 2s and 2p subshells are completely degenerate in a hydrogen atom.
- Answer
-
The hydrogen atom has only one electron; thus there is no shielding to consider. When there are not other electrons to shield the nucleus, penetration and shielding are irrelevant, and subshells within a shell are degenerate.
Which electrons shield others more effectively: 3p or 3d?
- Answer
-
3p shields better than 3d because p orbitals penetrate more than d orbitals within the same shell.
Explain why the ground state (most energetically favorable) electron configuration of Be is 1s22s2 rather than alternative configurations like 1s22s12p1 or 1s22p2.
- Answer
-
This question is asking why the 2s orbital fills in Be before 2p is occupied. This is a multi-electron atom, therefore core electrons shield the 2s and 2p orbitals to different extents. In Be we expect the 2s orbital to fill before 2p because 2s penetrates more and experiences a higher Z*.
References
- Petrucci, Ralph H., William S. Harwood, F. Geoffrey Herring, and Jeffry D. Madura. General Chemistry: Principles and Modern Applications, Ninth Edition. Pearson Education Inc. Upper Saddle River, New Jersey: 2007.
- Raymond Chang. Physical Chemistry for Biological Sciences. Sausalito, California: University Science Books, 2005
- R. S. Mulliken, Electronic Structures of Molecules and Valence. II General Considerations, Physical Review, vol. 41, pp. 49-71 (1932)
- Anastopoulos, Charis (2008). Particle Or Wave: The Evolution of the Concept of Matter in Modern Physics. Princeton University Press. pp. 236–237. ISBN 0691135126. http://books.google.com/?id=rDEvQZhpltEC&pg=PA236.
Contributors and Attributions
- Sidra Ayub (UCD), Alan Chu (UCD)
- Emily V Eames (City College of San Francisco)
- Kathryn Haas (Duke)

