4.E: Stoichiometry of Chemical Reactions- Homework
- Page ID
- 428710
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)For Chapter 4 you MUST Know
-
Solubility rules for:
- Always Soluble: lithium, sodium, potassium, ammonium, acetate, and nitrate
- Usually Soluble with exceptions for silver (I) and lead (II)
- chloride, bromide, iodide
- sulfate with additional exceptions for calcium, strontium, and barium
- Usually NOT soluble with exceptions for ammonium and alkali metals
- carbonate
- phosphate
- hydroxides added exceptions for strontium, and barium
Turn in your answers for the following questions - show your work
- Balance the following equations. Give the names of the reactants and the products. Given 10.0 grams of each reactant. How many grams of each product could be produced? What is the total mass of reactants and products when the reaction is complete?
- CH4 (g) + O2 (g) --> CO2 (g) + H2O (g)
- NaCl (aq) + AgNO3 (aq) --> AgCl (s) + NaNO3 (aq)
- Solution Reaction
- 10.00 grams of solid potassium hydroxide is added to 250.0 mL of distilled water. Write a chemical equation that describes what happens. Calculate the moles of each product.
- 10.00 grams of pure hydrochloric acid is added to 250.0 mL of distilled water. Write a chemical equation that describes what happens. Calculate the moles of each product.
- The two solutions are mixed together.
- Write a chemical equation that describes what happens.
- Calculate the moles of each product.
- Calculate the molarity of each product.
- Solution Reaction
- 5.00 grams of sodium chloride is diluted to 100.0 mL with distilled water. Write a chemical equation that describes what happens, calculate the concentration of each ion in solution, calculate the mass of any solid.
- 5.00 grams of silver (I) nitrate is diluted to 100.0 mL with distilled water. Write a chemical equation that describes what happens, calculate the concentration of each ion in solution, and calculate the mass of any solid.
- The two solutions above are mixed together.
- Write a chemical equation that describes what happens
- Calculate the moles of each ion in the final solution
- Calculate the mass of any solid product
- Calculate the molarity of each ion in the final solution
The Following Questions are for your practice - Do Not Turn In. They include answers so you can check your work
Writing and Balancing Chemical Equations
- Write a balanced molecular equation describing each of the following chemical reactions.
- Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas.
- Gaseous butane, C4H10, reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor.
- Answer a
- \(\ce{CaCO3}(s)\rightarrow \ce{CaO}(s)+\ce{CO2}(g)\);
- Answer b
- \(\ce{2C4H10}(g)+\ce{13O2}(g)\rightarrow \ce{8CO2}(g)+\ce{10H2O}(g)\);
- From the balanced molecular equations, write the complete ionic and net ionic equations for the following:
- \(\ce{K2C2O4}(aq)+\ce{Ba(OH)2}(aq)\rightarrow \ce{2KOH}(aq)+\ce{BaC2O2}(s)\)
- answer
- \[\ce{2K+}(aq)+\ce{C2O4^2-}(aq)+\ce{Ba^2+}(aq)+\ce{2OH-}(aq)\rightarrow \ce{2K+}(aq)+\ce{2OH-}(aq)+\ce{BaC2O4}(s)\hspace{20px}\ce{(complete)}\] \[\ce{Ba^2+}(aq)+\ce{C2O4^2-}(aq)\rightarrow \ce{BaC2O4}(s)\hspace{20px}\ce{(net)}\]
Classifying Chemical Reactions
Complete and balance the equations for the following acid-base neutralization reactions. If water is used as a solvent, write the reactants and products as aqueous ions. In some cases, there may be more than one correct answer, depending on the amounts of reactants used.
-
- \(\ce{Mg(OH)2}(s)+\ce{HClO4}(aq)\rightarrow \)
- \(\ce{SO3}(g)+\ce{H2O}(l)\rightarrow \) (assume an excess of water and that the product dissolves)
- answer a
- \(\ce{Mg(OH)2}(s)+\ce{2HClO4}(aq)\rightarrow \ce{Mg^2+}(aq)+\ce{2ClO4-}(aq)+\ce{2H2O}(l)\) ;
- answer b
- \(\ce{SO3}(g)+\ce{2H2O}(l)\rightarrow \ce{H3O+}(aq)+\ce{HSO4-}(aq)\), (a solution of H2SO4);
Reaction Stoichiometry
- H2 is produced by the reaction of 118.5 mL of a 0.8775-M solution of H3PO4 according to the following equation: \(\ce{2Cr + 2H3PO4 \rightarrow 3H2 + 2CrPO4}\).
- Outline the steps necessary to determine the number of moles and mass of H2.
- Answer
-
- Convert mL to L
- Multiply L by the molarity to determine moles of H3PO4
- Convert moles of H3PO4 to moles of H2
- Multiply moles of H2 by the molar mass of H2 to get the answer in grams
- Perform the calculations outlined.
- Answer
-
1. \(118.5\: mL\times \dfrac{1\: L}{1000\: mL} = 0.1185\: L\)
2. \(0.1185\: L \times \dfrac{0.8775\: moles\: \ce{H3PO4}}{1\: L} = 0.1040\: moles\: \ce{H3PO4}\)
3. \(0.1040\: moles\: \ce{H3PO4} \times \dfrac{3\: moles\:\ce{H_2}}{2\: moles\: \ce{H3PO4}} = 0.1560\: moles\: \ce{H2}\)
4. \(0.1560\: moles\: \ce{H2} \times \dfrac{2.02 g}{1\: mole} = 0.3151g\: \ce{H2}\)
- Outline the steps necessary to determine the number of moles and mass of H2.
- Carborundum is silicon carbide, SiC, a very hard material used as an abrasive on sandpaper and in other applications. It is prepared by the reaction of pure sand, SiO2, with carbon at high temperature. Carbon monoxide, CO, is the other product of this reaction. Write the balanced equation for the reaction, and calculate how much SiO2 is required to produce 3.00 kg of SiC.
- Answer
- \(\ce{SiO2 + 3C \rightarrow SiC + 2CO}\), 4.50 kg SiO2
- Urea, CO(NH2)2, is manufactured on a large scale for use in producing urea-formaldehyde plastics and as a fertilizer. What is the maximum mass of urea that can be manufactured from the CO2 produced by combustion of 1.00×103 kg of carbon followed by the reaction?
\[\ce{CO2}(g)+\ce{2NH3}(g)\rightarrow \ce{CO(NH2)2}(s)+\ce{H2O}(l)\]
- Answer
- 5.00 × 103 kg
Reaction Yields
- Freon-12, CCl2F2, is prepared from CCl4 by reaction with HF. The other product of this reaction is HCl. Outline the steps needed to determine the percent yield of a reaction that produces 12.5 g of CCl2F2 from 32.9 g of CCl4. Freon-12 has been banned and is no longer used as a refrigerant because it catalyzes the decomposition of ozone and has a very long lifetime in the atmosphere. Determine the percent yield.
- Answer
- \(\ce{g\: CCl4\rightarrow mol\: CCl4\rightarrow mol\: CCl2F2 \rightarrow g\: CCl2F2}, \mathrm{\:percent\: yield=48.3\%}\)
- How many molecules of the sweetener saccharin can be prepared from 30 C atoms, 25 H atoms, 12 O atoms, 8 S atoms, and 14 N atoms?
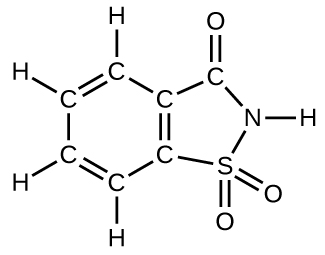
- Answer
- Only four molecules can be made.
Quantitative Chemical Analysis
- Titration of a 20.0-mL sample of acid rain required 1.7 mL of 0.0811 M NaOH to reach the end point. If we assume that the acidity of the rain is due to the presence of sulfuric acid, what was the concentration of sulfuric acid in this sample of rain?
- Answer
- 3.4 × 10−3 M H2SO4
- A 0.025-g sample of a compound composed of boron and hydrogen, with a molecular mass of ~28 amu, burns spontaneously when exposed to air, producing 0.063 g of B2O3. What are the empirical and molecular formulas of the compound.
- Answer
- The empirical formula is BH3. The molecular formula is B2H6.


