3.E: Composition of Substances and Solutions- Homework
- Page ID
- 428698
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
For Chapter 3 you MUST know:
- The additional polyatomic ions: cyanide ( \(\ce{CN^-}\)), nitrite ( \(\ce{NO_2^-}\)), and sulfite ( \(\ce{SO_3^{2-}}\)).
Turn in your answers for the following questions - show your work
- For the following molecules; write the chemical formula, determine the molecular weight and determine the number of moles in exactly 1 gram.
- carbon dioxide
- iron (II) chloride
- dinitrogen pentoxide
- iron (III) sulfate
- Name the following compounds, determine the molecular weight and determine how many moles in 8.35 grams of the compound.
- KI
- CaF2
- Cu2SO4
- N2O
- LiOH
- Give the chemical formula (or atomic symbol), molecular (or atomic) weight, and charge for the following ions:
- sulfate
- sulfite
- nitrate
- chloride
- nitride
- acetate
- carbonate
- Calculate the number of moles in:
- 45.3594 g of Ne
- 0.198669 g of Ne
- Calculate the mass of:
- 2.00 mole of Fe
- 4.362 x 10-5 mol of Fe
- Describe how to prepare 250 mL of 0.12 M lithium chloride solution from solid lithium chloride.
- Describe how to prepare 100 mL of 0.012 M lithium chloride solution from a 0.12 M lithium chloride solution.
The Following Questions are for your practice - Do Not Turn In. They include answers so you can check your work
3.1: Formula Mass and the Mole Concept
- What is the total mass (amu) of carbon in each of the following molecules?
- CH4
- C12H10O6
- (a)
- 12.01 amu
- (b)
- 144.12 amu
- Calculate the molecular or formula mass of each of the following:
- P4
- H2O
- Ca(NO3)2
- (a)
- 123.896 amu
- (b)
- 18.015 amu
- (c)
- 164.086 amu
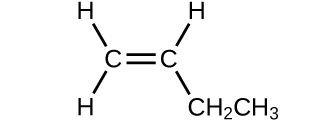
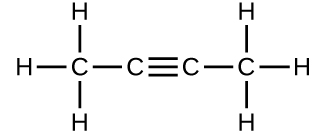
- Determine the molecular mass of the following compounds:
- (a)
- 56.107 amu
- (b)
- 54.091 amu
- Calculate the molar mass of each of the following:
- S8
- C5H12
- (a)
- 256.528 g/mol
- (b)
- 72.150 g mol−1
- Determine the mass of each of the following:
- 0.0146 mol KOH
- 10.2 mol ethane, C2H6
- (a)
- 0.819 g
- (b)
- 307 g
3.2: Determining Empirical and Molecular Formulas
- answer
- Mg3Si2H3O8 (empirical formula), Mg6Si4H6O16 (molecular formula)
- Calculate the percent composition of ammonia, NH3 to four significant figures:
- answer
- % N = 82.24%; % H = 17.76%
- A compound of carbon and hydrogen contains 92.3% C and has a molar mass of 78.1 g/mol. What is its molecular formula?
- answer
- C6H6
- Determine the empirical and molecular formula for chrysotile asbestos. Chrysotile has the following percent composition: 28.03% Mg, 21.60% Si, 1.16% H, and 49.21% O. The molar mass for chrysotile is 520.8 g/mol.
3.3: Molarity
- Determine the molarity for 0.444 mol of CoCl2 in 0.654 L of solution
- answer
- 0.679 M
- Determine the molarity for 98.0 g of phosphoric acid, H3PO4, in 1.00 L of solution
- answer
- 1.00 M
- Calculate the number of moles and the mass of the solute in 2.00 L of 18.5 M H2SO4, concentrated sulfuric acid:
- answer
- 37.0 mol H2SO4; 3.63 × 103 g H2SO4
- Calculate the molarity of 0.195 g of cholesterol, C27H46O, in 0.100 L of serum, the average concentration of cholesterol in human serum
- answer
- 5.04 × 10−3 M
- What is the molarity of the diluted solution when 1.00 L of a 0.250-M solution of Fe(NO3)3 is diluted to a final volume of 2.00 L?
- answer
- 0.125 M
- What volume of a 0.20-M K2SO4 solution contains 57 g of K2SO4?
- answer
- 1.6 L