3.E: Thermochemistry (Exercises)
- Page ID
- 393513
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)3.1: The Nature of Energy
If a person exerts a force on an immovable object, does that person do work? Explain your answer.
- Answer
-
Technically, the person is not doing any work, since the object does not move.
Why does hammering a piece of sheet metal cause the metal to heat up?
- Answer
-
The kinetic energy of the hammer is transferred to the metal.
Describe the mathematical relationship between (a) the thermal energy stored in an object and that object’s mass and (b) the thermal energy stored in an object and that object’s temperature.
- Answer
-
a. The thermal energy content of an object is directly proportional to its mass.
b. The thermal energy content of an object is directly proportional to its temperature.
Calculate how much energy (in kilojoules) is released or stored when each of the following occurs:
a. A 130 lb ice skater is lifted 7.50 ft off the ice.
b. 48 lb child jumps from a height of 4.0 ft.
c. An 18.5 lb light fixture falls from a 10.0 ft ceiling.
- Answer
-
a. 1.3 kJ stored
b. 0.26 kJ released
c. 0.251 kJ released
3.2: The First Law of Thermodynamics
What is the relationship between enthalpy and internal energy for a reaction that occurs at constant pressure?
- Answer
-
At constant pressure, ΔH = ΔU + PΔV
An intrepid scientist placed an unknown salt in a small amount of water. All the salt dissolved in the water, and the temperature of the solution dropped several degrees.
- What is the sign of q for this reaction?
- What is the sign of the enthalpy change for this reaction?
- Is the dissolution exothermic or endothermic?
- Answer
-
a. The temperature of the solution dropped, so the solution lost heat. That means the reaction gained heat so q for the reaction is positive.
b. Since the q of the reaction is positive, the sign of the enthalpy change is also positive.
c. Since heat is transferred from the surroundings to the system, it is endothermic.
3.3 & 3.4: Enthalpy
Heat implies the flow of energy from one object to another. Describe the energy flow in an
a. exothermic reaction.
b. endothermic reaction.
- Answer
-
a. In an exothermic reaction heat energy flows from the sytem to the surroundings
b. In an endothermic reaction heat energy flows from the surroundings to the system.
In each scenario, the system is defined as the mixture of chemical substances that undergoes a reaction. State whether each process is endothermic or exothermic.
a. Water is added to sodium hydroxide pellets, and the flask becomes hot.
b. Ammonium nitrate crystals are dissolved in water, causing the solution to become cool.
- Answer
-
a. exothermic
b. endothermic
Given the following thermochemical equation, what is ΔH for the reverse reaction?
N2 (g) + 3H2 (g) → 2NH3 (g) ΔH = -91.8 kJ/mol
- Answer
-
ΔH = 91.8 kJ/mol
Given the following thermochemical equation,
C6H6 (l) → 3C2H2 (g) ΔH = 630.0 kJ/mol
a. Write the complete thermochemical equation for the reverse reaction. Sketch an enthalpy diagram showing the relative positions of all species and include arrows indicating both of the above reactions with appropriate values for ΔH reaction.
b. what is ΔH for this reaction?
6C2H2 (g) → 2C6H6 (l) ΔH = ?
- Answer
-
b. -1260.0 kJ/mol
Given the following thermochemical equation,
C6H6 (l) → 3C2H2 (g) ΔH = 630.0 kJ/mol
what is ΔH when 0.50 mole of C6H6 (l) reacts to form C2H2 (g)?
- Answer
-
315.0 kJ/mol
3.5: Calorimetry
How much heat is required to heat a 28.4-g ice cube from −23.0 °C to −1.0 °C? Use ice cp = 2.05 J/gK
- Answer
-
1.3 kJ
Using the data below, how much heat (q) is needed to raise the temperature of a 2.50 g piece of copper wire from 20.0 °C to 80.0 °C? How much heat is needed to increase the temperature of an equivalent mass of aluminum by the same amount?
Cu cp = 0.386 J/gK
Al cp = 0.900 J/gK
- Answer
-
For Cu: q = 57.9 J
For Al: q = 135 J
- Solution
-
ΔT = Tf - Ti = 80.0 °C - 20.0 °C = 60.0 °C = 60.0 K
q = cp mass ΔT
For Cu: q = 0.386 J/gK(2.50 g)(60.0 K) = 57.9 J
For Al: q = 0.900 J/gK(2.50 g)(60.0 K) = 135 J
Since mass and change in temperature are the same, it is the value of the specific heat capacity that affects q for the two metals. Since Al has a larger specific heat capacity, we would expect that it would take more heat to cause the same change intemperate of Al than Cu.
In an exothermic reaction, how much heat would need to be evolved to raise the temperature of 150.0 mL of water 7.5°C?
- Answer
-
4.7 x 103 J = 4.7 kJ
- Solution
-
Use the density of water as 1.00 g/mL, therefore we have 150.0 g of water.
The specific heat capaity of water is 4.186 J/gK.
ΔT = 7.5 oC = 7.5 K
q = cp mass ΔT
q = 4.186 J/gK(150.0 g)(7.5 K) = 4.7 x 103 J = 4.7 kJ
Ethylene glycol, used as a coolant in automotive engines, has a specific heat capacity of 2.42 J g-1 K-1. Calculate q for the system when 3.65 x 103 g of ethylene glycol is cooled from 115.0°C to 85.0°C.
- Answer
-
q = -265 kJ
When 5.03 g of solid potassium hydroxide are dissolved in 100.0 mL of distilled water in a coffee-cup calorimeter, the temperature of the liquid increases from 23.0°C to 34.7°C. The density of water in this temperature range averages 0.9969 g/cm3. What is ΔHsoln (in kilojoules per mole)?
Assume that the calorimeter absorbs a negligible amount of heat and, because of the large volume of water, the specific heat of the solution is the same as the specific heat of pure water.
- Answer
-
ΔHsoln = -57.2 kJ/mol
When 0.50 moles of solid ammonium nitrate is dissolved in 60.0 g of water in a coffee cup calorimeter, the q of the surroundings is determined to be -12.5 kJ. What is the enthalpy change for the reaction in kJ/mol of NH4NO3?
- Answer
-
25 kJ/mol
Dissolving 3.0 g of CaCl2(s) in 150.0 g of water in a coffe cup calorimeter at 22.4 °C causes the temperature of the solution to rise to 25.8 °C. What is the approximate amount of heat involved in the dissolution, assuming the heat capacity of the resulting solution is 4.18 J/g °C? Is the reaction exothermic or endothermic?
- Answer
-
2.2 kJ; The heat produced shows that the reaction is exothermic.
The addition of 3.15 g of Ba(OH)2•8H2O to a solution of 1.52 g of NH4SCN in 100 g of water in a calorimeter caused the temperature to fall by 3.1 °C. Assuming the specific heat of the solution and products is 4.20 J/g °C, calculate the approximate amount of heat absorbed by the reaction, which can be represented by the following equation:
Ba(OH)2⋅8H2O(s) + 2NH4SCN(aq) → Ba(SCN)2(aq) + 2NH3(aq) + 10H O(l)
- Answer
-
1.4 kJ
When a 0.740-g sample of trinitrotoluene (TNT), C7H5N2O6, is burned in a bomb calorimeter, the temperature increases from 23.4 °C to 26.9 °C. The heat capacity of the calorimeter is 534 J/°C, and it contains 675 mL of water. How much heat was produced by the combustion of the TNT sample?
- Answer
-
11.7 kJ
3.6: Hess's Law
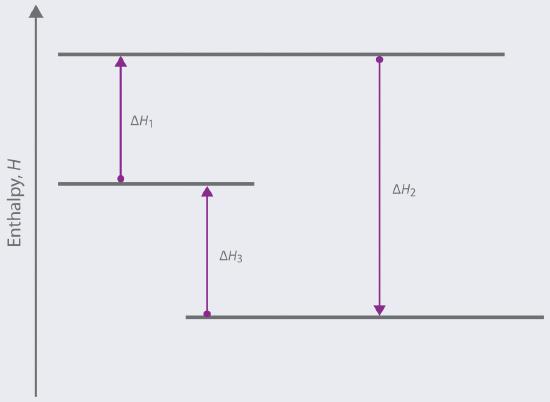
Based on the following energy diagram,
a. write an equation showing how the value of ΔH2 could be determined if the values of ΔH1 and ΔH3 are known.
b. identify each step as being exothermic or endothermic.
- Answer
-
a. ΔH2 = -ΔH1 + (-ΔH3)
b. -ΔH1 is exothermic since it is the reverse of ΔH1 which is endothermic (we know this since the final H is greater than the initial H following the direction of the arrow). -ΔH3 is exothermic for the same reason.
The following sequence of reactions occurs produces aqueous nitric acid:
4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(l) ΔH=−907 kJ
2NO(g) + O2(g) → 2NO2(g) ΔH=−113 kJ
3NO2 + H2O(l) → 2HNO3(aq) + NO(g) ΔH=−139 kJ
The overall reaction is:
\begin{equation}
4 \mathrm{NH}_3(\mathrm{~g})+6 \mathrm{O}_2(\mathrm{~g})+\mathrm{NO}_3(\mathrm{~g}) \rightarrow 2 \mathrm{HNO}_3(\mathrm{aq})+3 \mathrm{NO}(\mathrm{g})+5 \mathrm{H}_3 \mathrm{O}(\mathrm{l})
\end{equation}
Determine the total enthalpy change for the production of one mole of aqueous nitric acid by this overall reaction.
- Answer
-
-580. kJ/mol
Calculate ΔH for the process
Hg2Cl2(s) → 2Hg(l) + Cl2(g)
from the following information:
Hg(l) + Cl2(g) → HgCl2(s) ΔH=−224kJ
Hg(l) + HgCl2(s) → Hg2Cl2(s) ΔH=−41.2kJ
- Answer
-
265 kJ
Calculate ΔH for the process Zn(s) + S(s) + 2O2(g) → ZnSO4(s)
from the following information:
Zn(s) + S(s) → ZnS(s) ΔH=−206.0kJ
ZnS(s) + 2O2(g) → ZnSO4(s) ΔH=−776.8kJ
- Answer
-
-982.8 kJ
a. Use the enthalpies of combustion given in the following table to determine which organic compound releases the greatest amount of energy per gram during combustion.
| Fuel | ΔHocombustion(kJ/mol) |
|---|---|
| methanol | −726.1 |
| 1-ethyl-2-methylbenzene | −5210.2 |
| n-octane | −5470.5 |
b. Calculate the standard enthalpy of formation of 1-ethyl-2-methylbenzene.
- Answer
-
a.
methanol: ΔH/g = −22.6 kJ
C9H12: ΔH/g = −43.3 kJ
octane: ΔH/g = −47.9 kJ
Octane provides the largest amount of heat per gram upon combustion.
b. ΔHf(C9H17) = −46.1 kJ/mol
3.7: Enthalpies of Formation
Using data from table T1: Standard Thermodynamic Quantities, determine ΔHorxn for each chemical reaction.
a. 2Na(s) + Pb(NO3)2(s) → 2NaNO3(s) + Pb(s)
b. Na2CO3(s) + H2SO4(l) → Na2SO4(s) + CO2(g) + H2O(l)
c. 2KClO3(s) → 2KCl(s) + 3O2(g)
- Answer
-
a. −1203 kJ/mol O2
b. 179.2 kJ
c. −59.3 kJ
Use the data in table T1: Standard Thermodynamic Quantities to calculate ΔHorxn for the reaction
Sn(s, white) + 4HNO3(l) → SnO2(s) + 4NO2(g) + 2H2O(l).
- Answer
-
−174.1 kJ/mol
How much heat is released or required in the reaction of 0.50 mol of HBr(g) with 1.0 mol of chlorine gas to produce bromine gas? Use the data in table T1: Standard Thermodynamic Quantities.
- Answer
-
−20.3 kJ
In the mid-1700s, a method was devised for preparing chlorine gas from the following reaction:
NaCl(s) + H2SO4(l) + MnO2(s) → Na2SO4(s) + MnCl2(s) + H2O(l) + Cl2(g)
Use the data in table T1: Standard Thermodynamic Quantities and the given data below to calculate ΔH for this reaction. Is the reaction exothermic or endothermic?
Given: \(\Delta H_f^o\)H2SO4 (l) = -814.0 kj/mol
- Answer
-
−34.3 kJ/mol; exothermic