5: Lewis Structures
- Page ID
- 316200
Note: This PRE-LAB ASSIGNMENT MUST BE COMPLETED BEFORE COMING TO LAB. In your lab notebook, draw a large picture (Lewis structure) of all the molecules (such that you can write distances above the bond). You will use these structures label bond lengths and angles as you proceed through the experiment.
Learning Objectives
- To learn how to use one type of computer modeling
- To look at what affects bond lengths and bond angles.
Experiment:
We will be using a program called Spartan to construct and examine molecules in during lab.
Part I:
Construct the molecules in the table below using Spartan. Minimize each structure after constructing it, by using the Minimize button.  This must be completed after building each molecule.
This must be completed after building each molecule.
|
Bond Order |
Terminal Atoms |
Hetero Atoms |
|
CH4 |
CF4 |
H3CNH2 |
|
H3C – CH3 |
CCl4 |
H3COH |
|
H2C = CH2 |
CBr4 |
H3CPH2 |
|
HC ≡ CH |
CI4 |
H3CSH |
Step 1
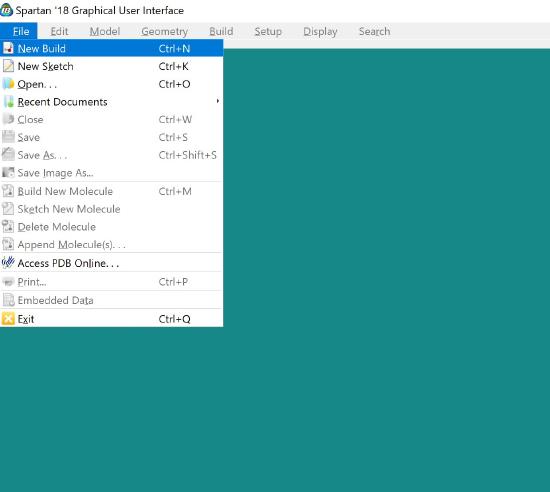 Open Spartan and click on new build.
Open Spartan and click on new build.
Step 2
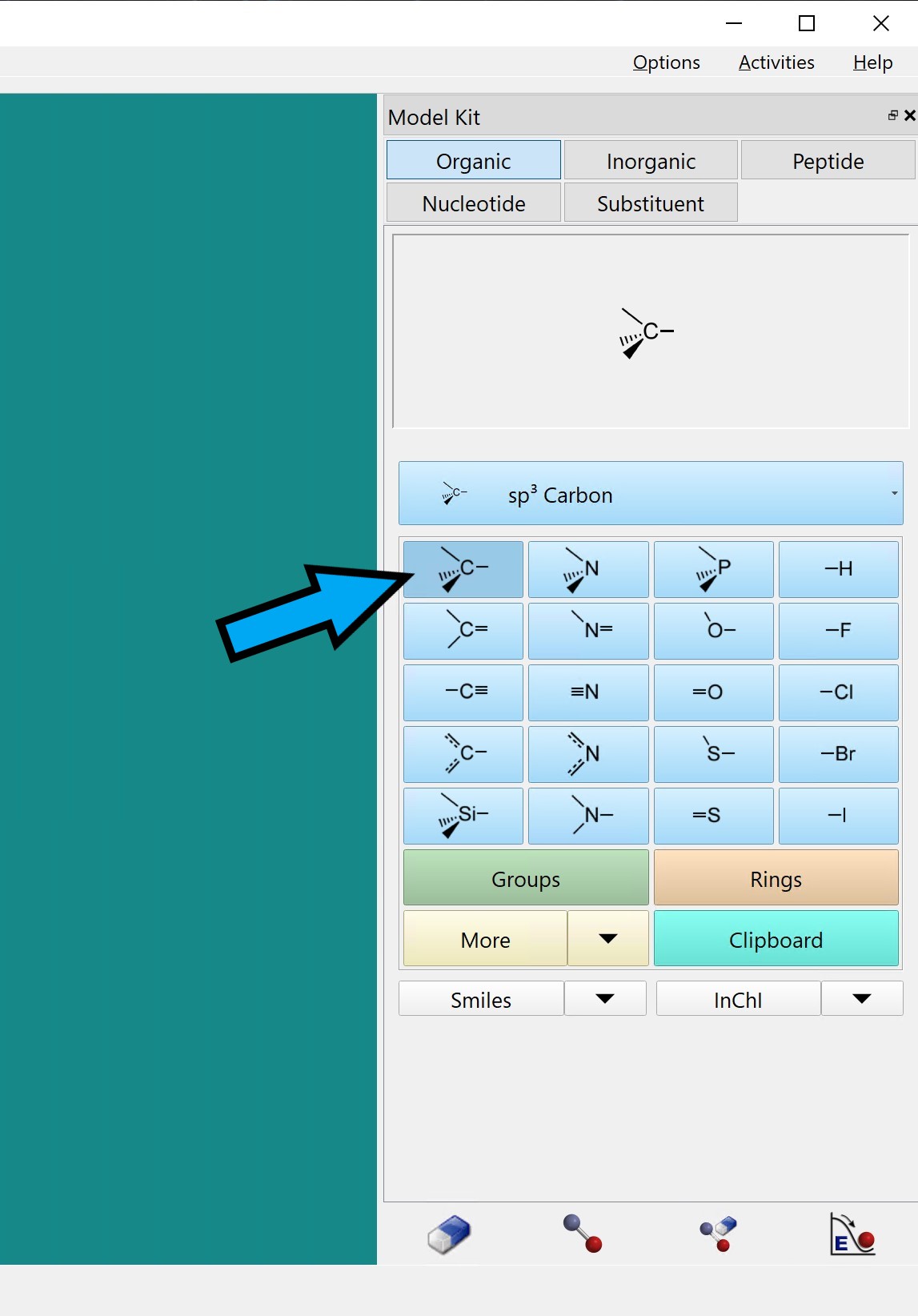 Click on the carbon atom with four bonds.
Click on the carbon atom with four bonds.
Step 3
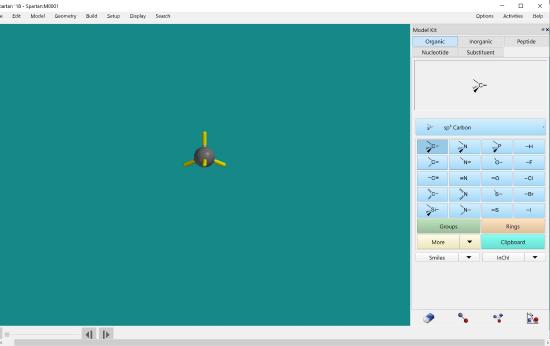 Double click on the screen. You will see a carbon atom with four bonds appear.
Double click on the screen. You will see a carbon atom with four bonds appear.
Step 4
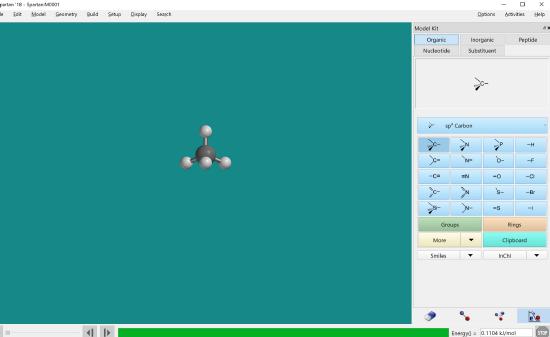 Click the minimize button. This will place hydrogen atoms on any open bonds. Reminder this must be completed after each and every molecule is built regardless if hydrogen needs to be added or not.
Click the minimize button. This will place hydrogen atoms on any open bonds. Reminder this must be completed after each and every molecule is built regardless if hydrogen needs to be added or not.
Step 5
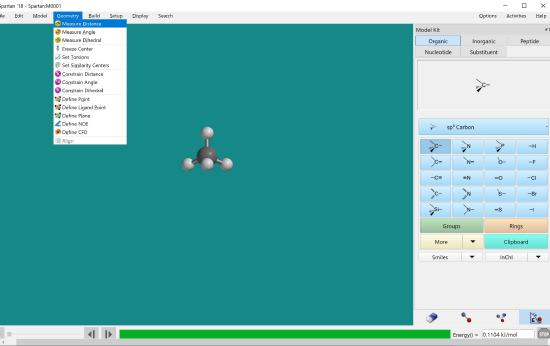 Click measure distance under the Geometry menu.
Click measure distance under the Geometry menu.
Step 6
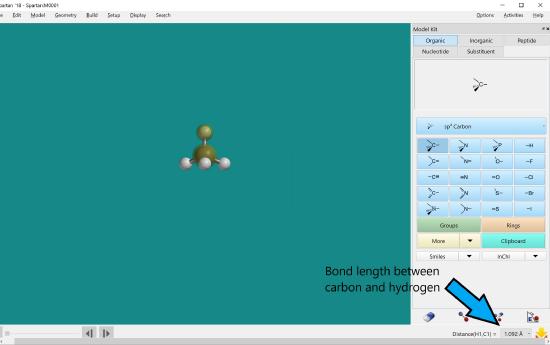 Click the two atoms on either side of the bond that you would like to measure or click on the bond. The distance measurement will be in the lower right hand corner.
Click the two atoms on either side of the bond that you would like to measure or click on the bond. The distance measurement will be in the lower right hand corner.
Step 7
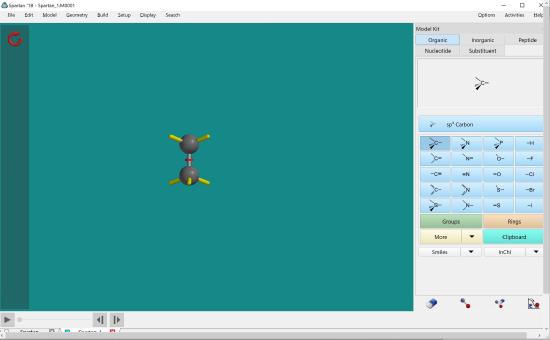 To create a bond between two carbons, double click the end of one of the bonds.
To create a bond between two carbons, double click the end of one of the bonds.
Step 8
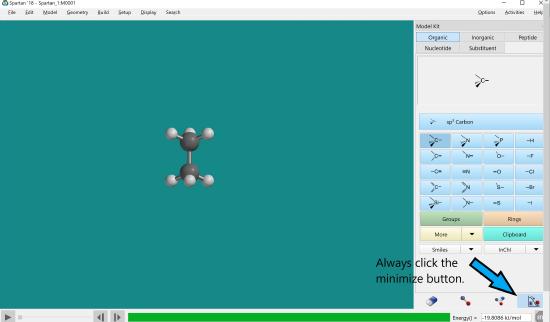 Click the minimize button. Measure bond lengths.
Click the minimize button. Measure bond lengths.
Step 9
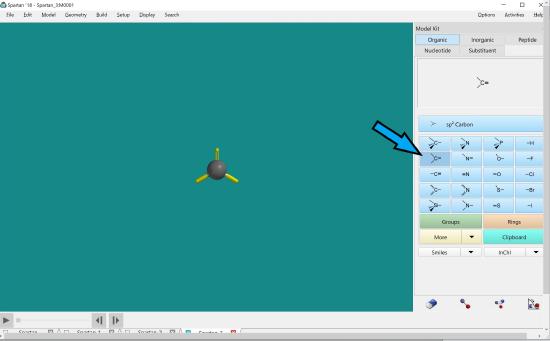 To create a double bond between two carbon atoms, click the carbon with the double bond in the atom menu. Double click on the screen.
To create a double bond between two carbon atoms, click the carbon with the double bond in the atom menu. Double click on the screen.
Step 10
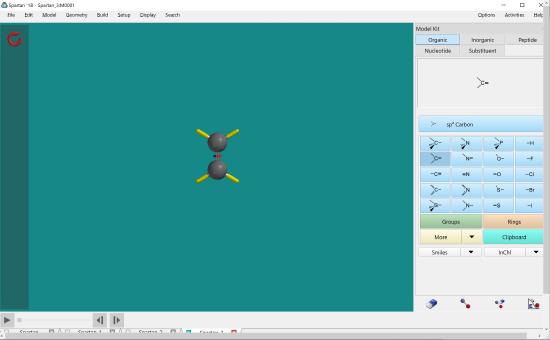 To attach the second carbon atom, double click the end of the double bond.
To attach the second carbon atom, double click the end of the double bond.
Step 11
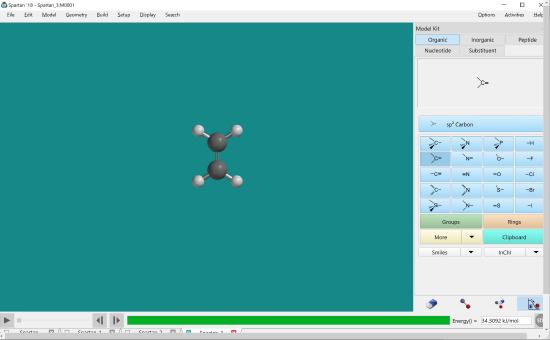 Click minimize. Measure bond lengths.
Click minimize. Measure bond lengths.
Step 12
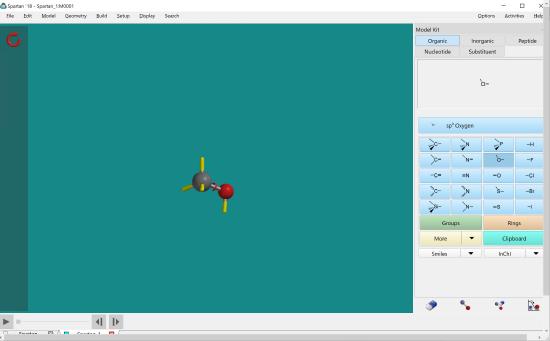 To attach a different atom to a carbon, first click the carbon with four bonds and then double click on the screen. Then click the atom you would like to attach in the atom menu and double click the end of one of the bonds on the carbon that you just created on the screen.
To attach a different atom to a carbon, first click the carbon with four bonds and then double click on the screen. Then click the atom you would like to attach in the atom menu and double click the end of one of the bonds on the carbon that you just created on the screen.
Application of Part I:
Step 1
Using the methods discussed in the pre-lab and your data from Part I-1, predict the lengths of the following bonds. Explain your answers.
N-F, N-O, N-Cl, H-F
Step 2
Construct a molecule containing one or more of these bonds so that you can measure the bond lengths on Spartan. You must be certain that you can compare the bonds. This means that they must all have the same bond order (all single bonds). Do your predictions agree with Spartan?
Part II:
Construct the following molecules and measure all the bond angles in the molecule. Be sure that you select the atoms in the order of the angle being measured. i.e. H – C – H for CH4. Record these drawings and your bond angles in your notebook.
| CH4 | NH3 | H2O |
| H2C = CH2 | H2C = O | HC ≡ CH |
 |
Step 1
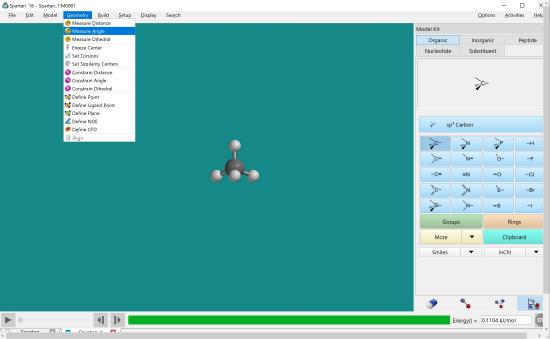 Build CH4 molecule. To measure the angle, click measure angle under the geometry menu.
Build CH4 molecule. To measure the angle, click measure angle under the geometry menu.
Step 2
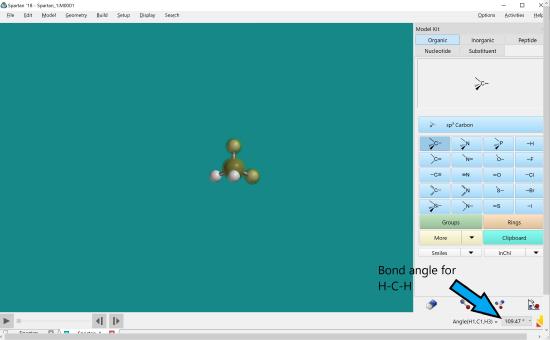 Click the atoms in the proper order, hydrogen then carbon followed by the last hydrogen in the bond angle to be measured. The bond angle will be in the lower right hand corner.
Click the atoms in the proper order, hydrogen then carbon followed by the last hydrogen in the bond angle to be measured. The bond angle will be in the lower right hand corner.
Step 3
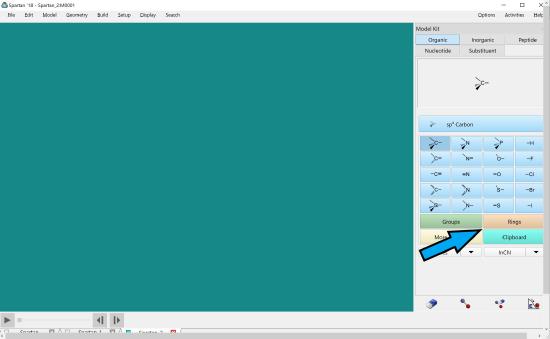 To build a benzene ring, click rings in the atom menu.
To build a benzene ring, click rings in the atom menu.
Step 4
 The benzene ring will appear in the top box.
The benzene ring will appear in the top box.
Step 5
 Double click on the screen. Click the minimize button and measure the angles of the carbon, carbon, carbon bond as well as the hydrogen, carbon, carbon bond.
Double click on the screen. Click the minimize button and measure the angles of the carbon, carbon, carbon bond as well as the hydrogen, carbon, carbon bond.
Part III:
Some practice on Lewis Structures
Complete the steps below for the molecules, NO2+ N2O NO2Cl NH2- Note: the underlined atom is the central atom.
Step 1
Draw the complete, correct Lewis structure
Step 2
Write all formal charges that are different from zero (0)
Step 3
Indicate the total number of electronic domains around the central or all internal atom(s) (underlined), specifying whether they are bonding or non-bonding.
Step 4
Predict the electronic and molecular shapes of the central atom in the ions and molecules below. It is up to you to determine if you use multiple bonding of the oxygen atoms to the nitrogen atom but you must explain your choices in your report.
Discussion:
- Summarize your data for the experiment in a table.
- What factors control the length of a bond?
- What factors control the angle of a bond?
- Did you see any relationship between bond order and bond angle?

