7.3: Isomers
- Page ID
- 207348
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Isomerism
Isomers are two or more compounds that have the same molecular formula, but have a different arrangement of atoms in a molecule. There are many different types of isomerism in organic molecules, we will review some of the different types here.
Example:
All of the hydrocarbons containing 4 or more carbon atoms show structural isomerism, meaning that there are two or more different structural formulae that you can draw for each molecular formula.
EXAMPLE: BUTANE OR METHYLPROPANE
C4H10 could be either of these two different molecules:
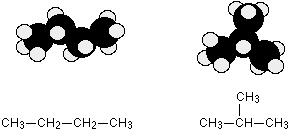
These are named butane and 2-methylpropane, respectively
What is structural isomerism?
Isomers are molecules that have the same molecular formula, but have a different arrangement of the atoms in space. That excludes any different arrangements which are simply due to the molecule rotating as a whole, or rotating about particular bonds. For example, both of the following are the same molecule. They are not isomers; both are butane.
There are also endless other possible ways that this molecule could twist itself (conformational isomers). There is completely free rotation around all the carbon-carbon single bonds. If you had a model of a molecule in front of you, you would have to take it to pieces and rebuild it if you wanted to make an isomer of that molecule. If you can make an apparently different molecule just by rotating single bonds, it's not different - it's still the same molecule.
In structural isomerism, the atoms are arranged in a completely different order. This is easier to see with specific examples. What follows looks at some of the ways that structural isomers can arise. The names of the various forms of structural isomerism probably do not matter all that much, but you must be aware of the different possibilities when you come to draw isomers.
Constitutional Isomers
Constitutional isomers have the same molecular formula and different connectivity of atoms. An example of this may be chain isomers: these isomers arise because of the possibility of branching in carbon chains. For example, there are two isomers of butane, C4H10. In one of them, the carbon atoms lie in a "straight chain" whereas in the other the chain is branched.
Be careful not to draw "false" isomers which are just twisted versions of the original molecule. For example, this structure is just the straight chain version of butane rotated about the central carbon-carbon bond.
You could easily see this with a model. This is the example we've already used at the top of this page.
EXAMPLE 3.2.13.2.1: CHAIN ISOMERS IN PENTANE
Pentane, C5H12, has three chain isomers. If you think you can find any others, they are simply twisted versions of the ones below. If in doubt make some models.
Exercises
Questions
Q3.2.1
Give all the isomers for a straight chain hexanol.
Solutions
S3.2.1
Geometric Isomers
Hydrocarbons with double bonds and other rigid structures introduce another type of isomers: geometric isomers. Geometic isomers have the same molecular formula, the same atomic connectivity, but different in arragment of atoms around a rigid structure. As an example we will look at 2-butene, an alkene. We give the name alkene to a hydrocarbon that has a double bond. Looking at an alkene with a four carbon chain where the double bond is placed between the two middle carbon atoms, there are two possible geometric isomers:
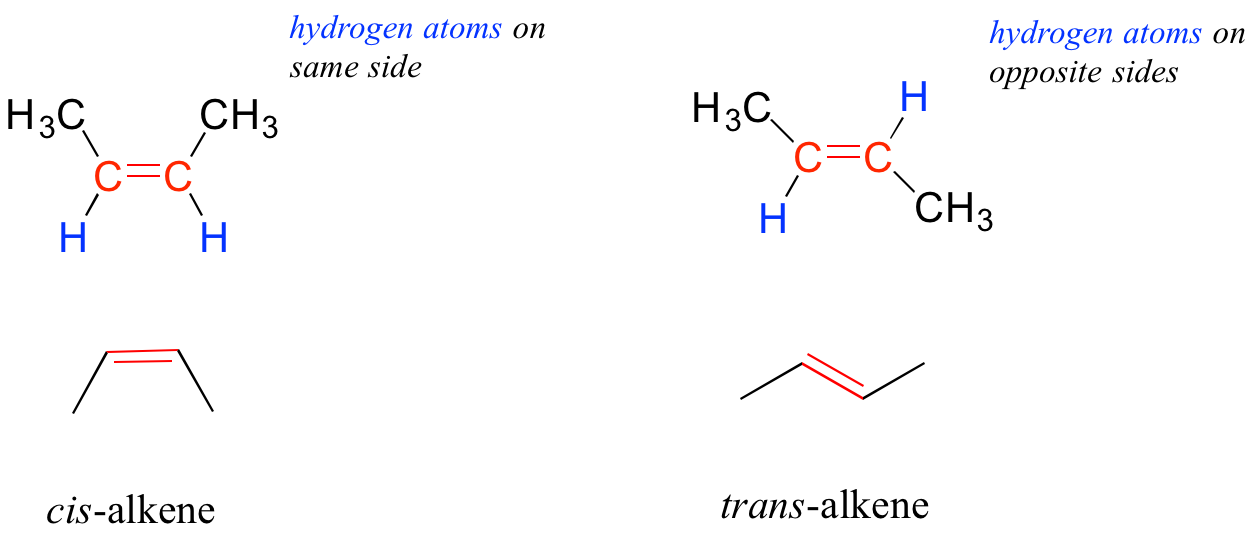
We use the term cis to denote, same side, the two CH3 groups are cis to one another in the structure on the left and the two CH3 groups are trans to one another on the structure on the right.
Chirality and Stereiosomers Isomers
Chirality and optical isomers are more challenging to understand than structural and geometric isomers. The term chiral means hand in latin, and a chiral object is one that displays "handness" or cannot be superimposed on its mirror image. Just like your right and left hand are mirror images of one another, but cannot be superimposed (or overlapped) with one another, there are molecules that are non-superimposable mirror images of one another. A molecule that exhibits this is considered chiral.
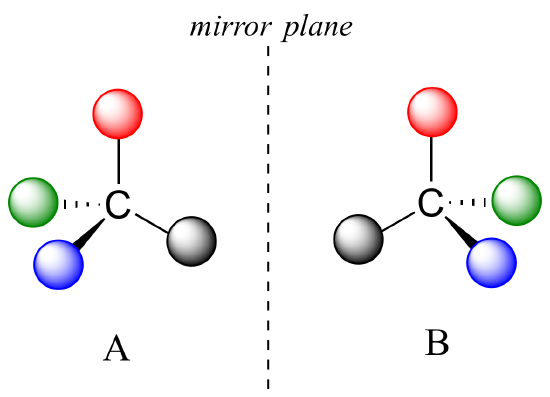
The mirror image of A, which we will call B, is drawn on the right side of the figure, and an imaginary mirror is in the middle. Notice that every point on A lines up through the mirror with the same point on B: in other words, if A looked in the mirror, it would see B looking back.
Now, if we flip compound A over and try to superimpose it point for point on compound B, we find that we cannot do it: if we superimpose any two colored balls, then the other two are misaligned.
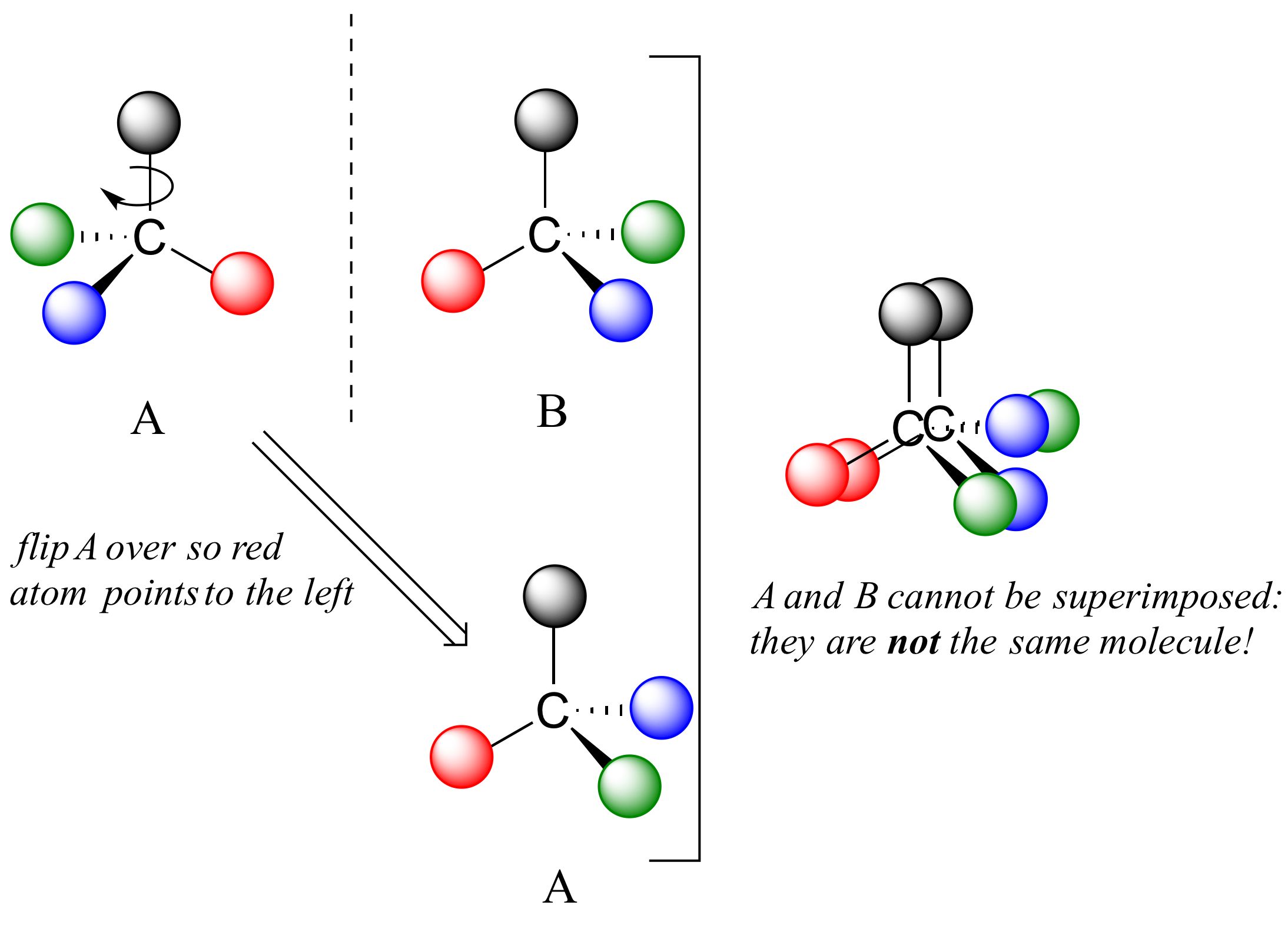
A is not superimposable on its mirror image (B), thus by definition A is a chiral molecule. It follows that B also is not superimposable on its mirror image (A), and thus it is also a chiral molecule. Also notice in the figure below (and convince yourself with models) that neither A nor B has an internal plane of symmetry.
A and B are stereoisomers: molecules with the same molecular formula and the same bonding arrangement, but a different arrangement of atoms in space.
Attributions: The material for this page was adapted from:
- 1.3: Functional groups and organic nomenclature and 3.4: Chirality and stereoisomers from the following text: Soderberg, Timothy, "Organic Chemistry with a Biological Emphasis Volume I" (2019). Chemistry Publications. 1. Textbook content licensed under the Creative Commons Attribution NonCommercial-Sharealike 4.0 license. Download for free at: https://digitalcommons.morris.umn.edu/chem_facpubs/1
- 3.2: Alkanes and Alkane Isomers from the following text: "Organic Chemistry LibreText (TextMap of McMurry's Book) Organic Chemistry" Textbook content licensed under the Creative Commons license.

