7.2: Representing Organic Compounds
- Page ID
- 207347
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Hydrocarbons and Heteroatoms
An organic compound is one in which one or more atoms of carbon are covlanently linked to other carbon atoms and/or linked to atoms of other elements (most commonly, hydrogen, oxygen and nitrogen). Carbon is special because it forms four bonds, has the capacity to form double and triple bonds and has a tendency to catenate, meaning it forms bonds with atoms of the same element. Organic molecules are primarily composed of carbon and hydrogens. In fact, some organic molecules only contain carbons and hydrogens: hydrocarbons are a common class of organic molecules that contain only carbon and hydrogen atoms, examples of hydrocarbons are alkanes, alkenes and alkynes. Non-carbon and hydrogen atoms are less common in organic compounds, and we give these atoms a special name: heteroatoms. A heteroatom is an atom that is not carbon or hydrogen.
Common bonding patterns in organic structures
The methods reviewed above for drawing Lewis structures and determining formal charges on atoms are an essential starting point for a novice organic chemist, and work quite will when dealing with small, simple structures. But as you can imagine, these methods become unreasonably tedious and time-consuming when you start dealing with larger structures. It would be unrealistic, for example, to ask you to draw the Lewis structure below (of one of the four nucleoside building blocks that make up DNA) and determine all formal charges by adding up, on an atom-by-atom basis, the valence electrons.
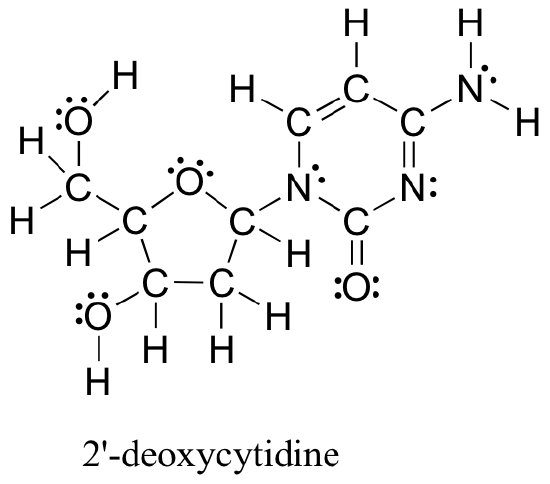
And yet, as organic chemists, and especially as organic chemists dealing with biological molecules, you will be expected soon to draw the structure of large molecules such as this on a regular basis. Clearly, you need to develop the ability to quickly and efficiently draw large structures and determine formal charges. Fortunately, this ability is not terribly hard to come by - all it takes is a few shortcuts and some practice at recognizing common bonding patterns.
Let’s start with carbon, the most important element for organic chemists. Carbon is said to be tetravalent, meaning that it tends to form four bonds. If you look at the simple structures of methane, methanol, ethane, ethene, and ethyne in the figures from the previous section, you should quickly recognize that in each molecule, the carbon atom has four bonds, and a formal charge of zero.
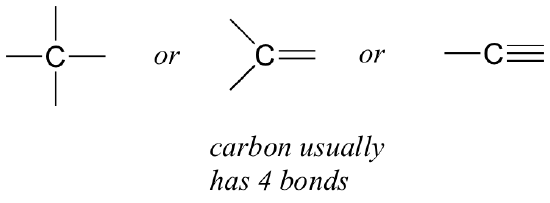
This is a pattern that holds throughout most of the organic molecules we will see, but there are also exceptions.
In carbon dioxide, the carbon atom has double bonds to oxygen on both sides (O=C=O). Later on in this chapter and throughout this book we will see examples of organic ions called ‘carbocations’ and carbanions’, in which a carbon atom bears a positive or negative formal charge, respectively. If a carbon has only three bonds and an unfilled valence shell (in other words, if it does not fulfill the octet rule), it will have a positive formal charge.
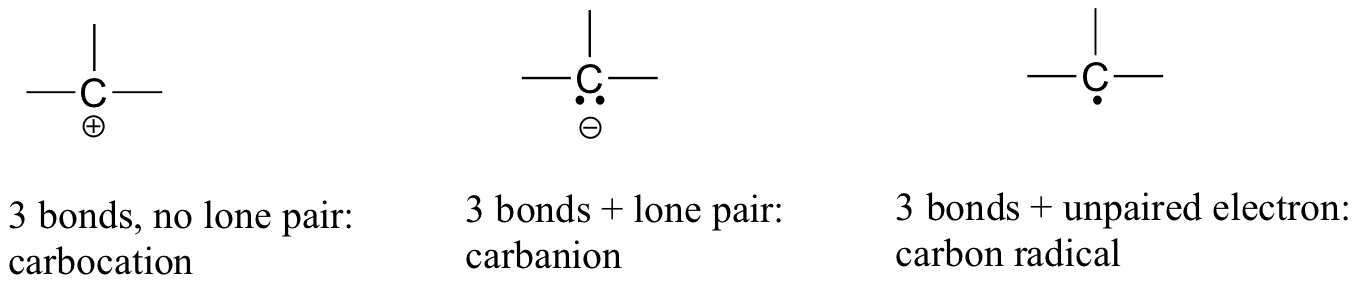
If, on the other hand, it has three bonds plus a lone pair of electrons, it will have a formal charge of -1. Another possibility is a carbon with three bonds and a single, unpaired (free radical) electron: in this case, the carbon has a formal charge of zero.
The pattern for hydrogens is easy: hydrogen atoms have only one bond, and no formal charge. The exceptions to this rule are the proton, H+, and the hydride ion, H-, which is a proton plus two electrons.
Let us next turn to oxygen atoms. Typically, you will see an oxygen bonding in three ways, all of which fulfill the octet rule.
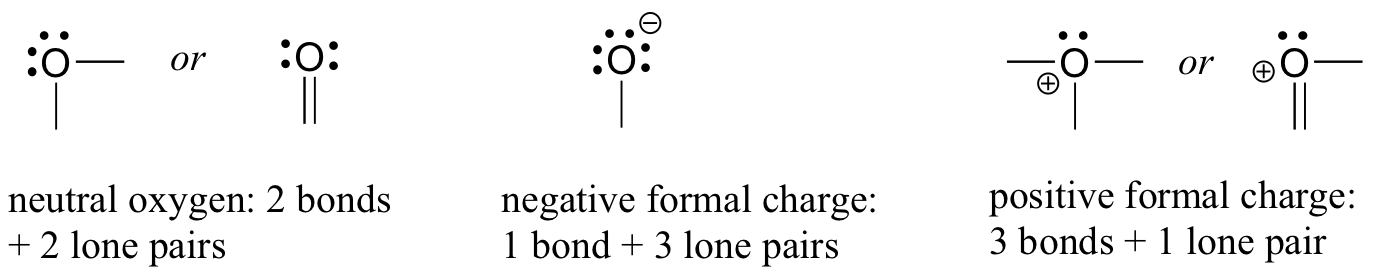
If an oxygen atom t has two bonds and two lone pairs, as in water, it will have a formal charge of zero. If it has one bond and three lone pairs, as in hydroxide ion, it will have a formal charge of-1. If it has three bonds and one lone pair, as in hydronium ion, it will have a formal charge of +1.
Nitrogen has two major bonding patterns, both of which fulfill the octet rule:
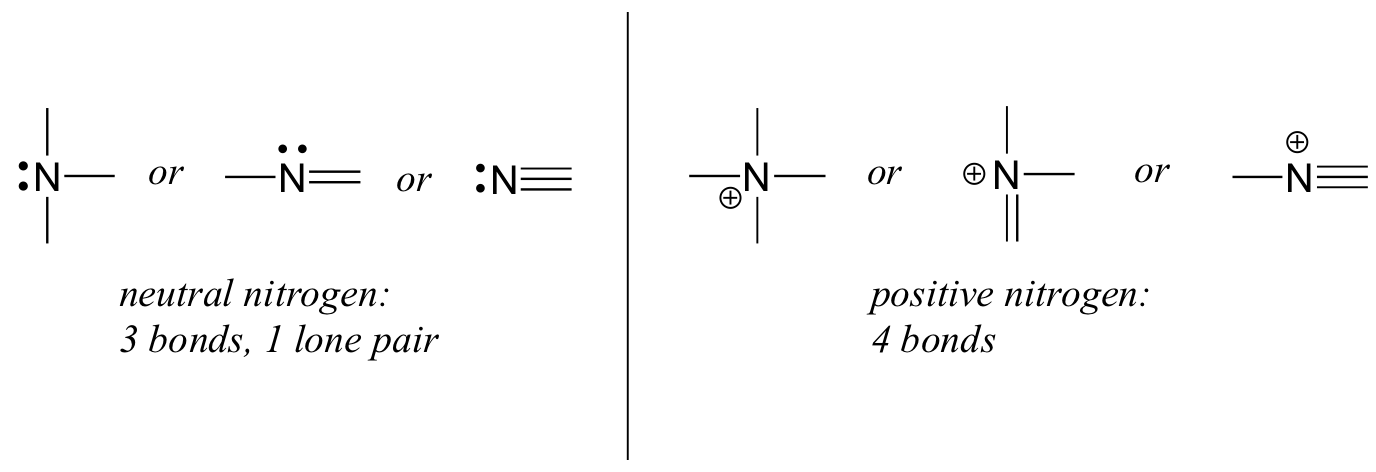
If a nitrogen has three bonds and a lone pair, it has a formal charge of zero. If it has four bonds (and no lone pair), it has a formal charge of +1. In a fairly uncommon bonding pattern, negatively charged nitrogen has two bonds and two lone pairs.
Using the 'line structure' convention
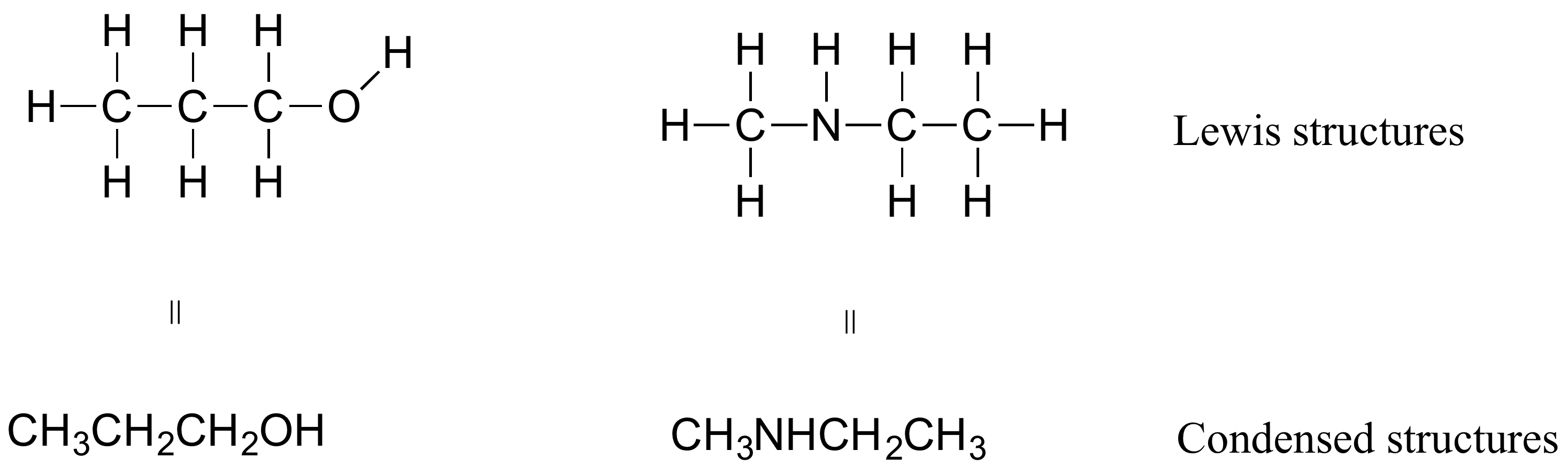
If you look ahead in this and other books at the way organic compounds are drawn, you will see that the figures are somewhat different from the Lewis structures you are used to seeing in your general chemistry book. In some sources, you will see condensed structures for smaller molecules instead of full Lewis structures:
More commonly, organic and biological chemists use an abbreviated drawing convention called line structures. The convention is quite simple and makes it easier to draw molecules, but line structures do take a little bit of getting used to. Carbon atoms are depicted not by a capital C, but by a ‘corner’ between two bonds, or a free end of a bond. Open-chain molecules are usually drawn out in a 'zig-zig' shape. Hydrogens attached to carbons are generally not shown: rather, like lone pairs, they are simply implied (unless a positive formal charge is shown, all carbons are assumed to have a full octet of valence electrons). Hydrogens bonded to nitrogen, oxygen, sulfur, or anything other than carbon are shown, but are usually drawn without showing the bond. The following examples illustrate the convention.
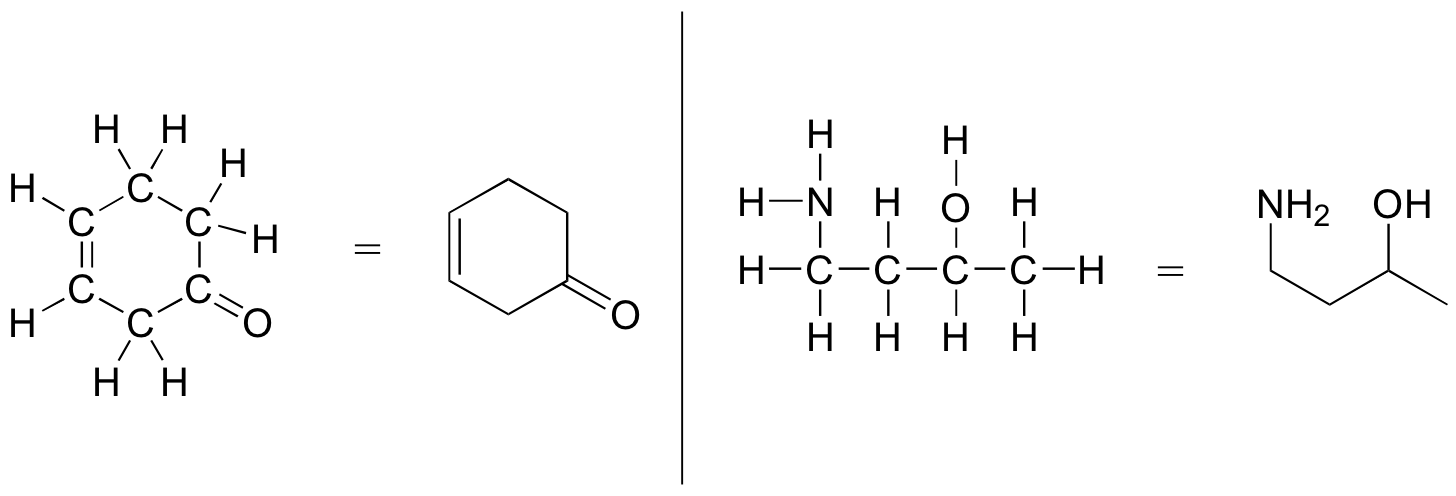
As you can see, the 'pared down' line structure makes it much easier to see the basic structure of the molecule and the locations where there is something other than C-C and C-H single bonds. For larger, more complex biological molecules, it becomes impractical to use full Lewis structures. Conversely, very small molecules such as ethane should be drawn with their full Lewis or condensed structures.
Sometimes, one or more carbon atoms in a line structure will be depicted with a capital C, if doing so makes an explanation easier to follow. If you label a carbon with a C, you also must draw in the hydrogens for that carbon.
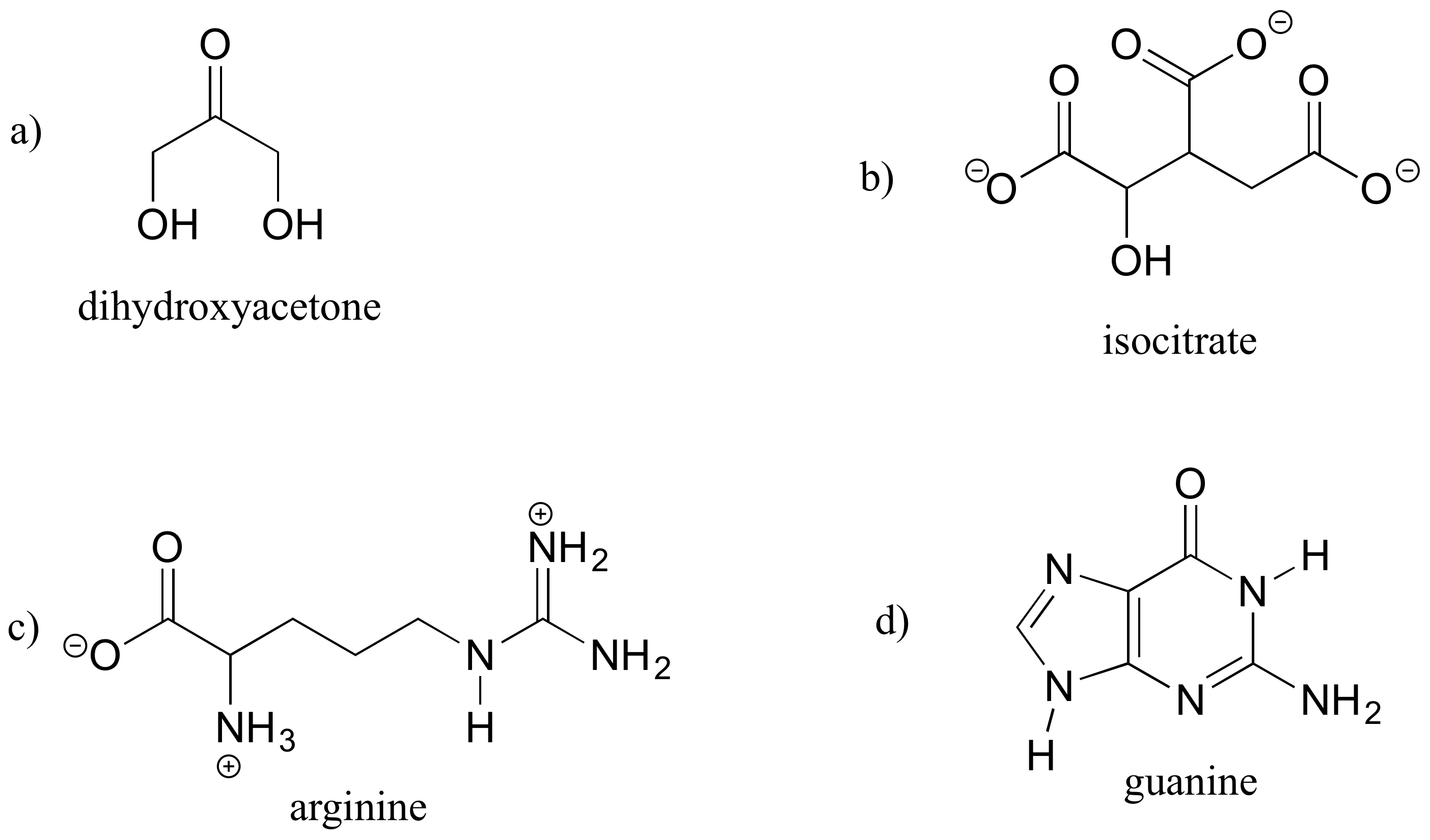
Exercise 1.6: A good way to test your understanding of the line structure convention is to determine the number of hydrogen atoms in a molecule from its line structure. Do this for the structures below.
Exercise 1.7:
a) Draw a line structure for the DNA base 2-deoxycytidine (the full structure was shown earlier)
b) Draw line structures for histidine (an amino acid) and pyridoxine (Vitamin B6).
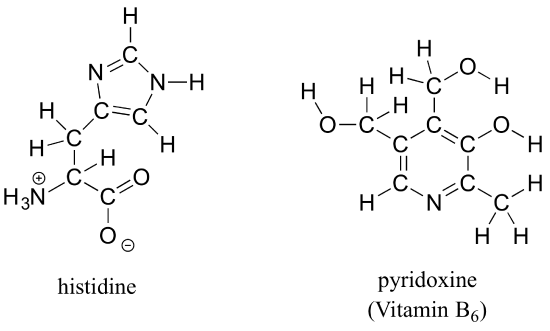
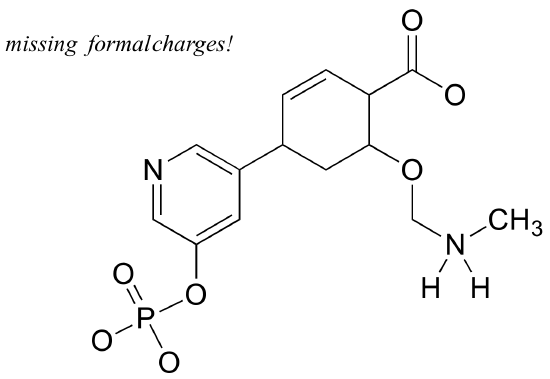
Exercise 1.8: Add non-zero formal charges to the structural drawing below. The net charge is -2.
Exercise 1.9: Find, anywhere in chapters 2-17 of this textbook, one example of each of the common bonding patterns specified below. Check your answers with your instructor or tutor.
a) carbon with one double bond, two single bonds, no lone pairs, and zero formal charge
b) oxygen with two single bonds, two lone pairs, and zero formal charge
c) oxygen with one double bond, two lone pairs, and zero formal charge
d) nitrogen with one double bond, two single bonds, and a +1 formal charge
e) oxygen with one single bond, three lone pairs, and a negative formal charge
Attributions: The material for this page was adapted from:
1.2: Drawing organic structures from the following text:
Soderberg, Timothy, "Organic Chemistry with a Biological Emphasis Volume I" (2019). Chemistry Publications. 1. Textbook content licensed under the Creative Commons Attribution NonCommercial-Sharealike 4.0 license. Download for free at: https://digitalcommons.morris.umn.edu/chem_facpubs/1

