8.5: Enzymes - Biological Catalysts
- Page ID
- 342727
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Outcomes
- Explain the role of an enzyme in the body.
- Define active site and substrate.
- Describe the lock and key vs. induced-fit model of enzymes.
- Provide the characteristics of a cofactor and a coenzyme.
All living cells carry out a great variety of chemical reactions essential for maintenance of life. Chemical reactions in living cells are different from chemical reactions in most other systems in that they are much faster and are highly ordered and regulated. The source of these differences is that the chemistry of living things is carried out by proteins functioning as specific biological catalysts, called enzymes. Enzymes accelerate and regulate biochemical processes allowing reactions to occur in the milliseconds necessary to maintain life.
The first enzyme to be isolated was discovered in 1926 by American chemist James Sumner, who crystallized the protein. The enzyme was urease, which catalyzes the hydrolytic decomposition of urea, a component of urine, into ammonia and carbon dioxide.
\[\ce{H_2NCON_2} \left( aq \right) + \ce{H_2O} \left( l \right) \overset{\text{urease}}{\rightarrow} 2 \ce{NH_3} \left( g \right) + \ce{CO_2} \left( g \right)\]
His discovery was ridiculed at first because nobody believed that enzymes would behave the same way that other chemicals did. Sumner was eventually proven right and won the Nobel Prize in Chemistry in 1946.
Enzyme-Catalyzed Reactions
Most chemical reactions within organisms would be impossible under the conditions in cells. For example, the body temperature of most organisms is too low for reactions to occur quickly enough to carry out life processes. Reactants may also be present in such low concentrations that it is unlikely they will meet and collide. Therefore, the rate of most biochemical reactions must be increased by a catalyst. A catalyst is a chemical that speeds up chemical reactions. In organisms, catalysts are called enzymes. Essentially, enzymes are biological catalysts.
Like other catalysts, enzymes are not reactants in the reactions they control. They help the reactants interact but are not used up in the reactions. Instead, they may be used over and over again. Unlike other catalysts, enzymes are usually highly specific for particular chemical reactions. They generally catalyze only one or a few types of reactions.
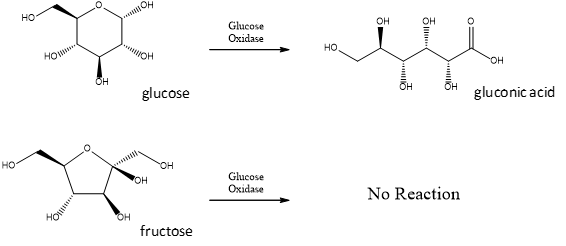
Figure \(\PageIndex{1}\): Enzymes catalyze specific reactions.
Enzymes are extremely efficient in speeding up reactions. They can catalyze up to several million reactions per second. As a result, the difference in rates of biochemical reactions with and without enzymes may be enormous. A typical biochemical reaction might take hours or even days to occur under normal cellular conditions without an enzyme, but less than a second with an enzyme.
The first step in an enzyme-catalyzed reaction is that the substrate binds to a specific part of the enzyme molecule. A substrate is the molecule(s) on which the enzyme acts. The binding of the substrate is dictated by the shape of each molecule. Side chains on the enzyme interact with the substrate in a specific way, resulting in the making and breaking of bonds. The active site is the specific part of an enzyme where the substrate binds. An enzyme folds in such a way that it typically has one active site, usually a pocket or crevice formed by the folding pattern of the protein. Because the active site of an enzyme has such a unique shape, only one particular substrate is capable of binding to that enzyme. This substrate specificity means that each enzyme catalyzes only one chemical reaction with only one substrate. Once the enzyme-substrate (ES) complex is formed, the reaction occurs and the substrate is transformed into products. Finally, the product molecule(s) are released from the active site. Note that the enzyme is left unaffected by the reaction and is now capable of catalyzing the reaction of another substrate molecule.

Figure \(\PageIndex{2}\): The sequence of steps for a substrate binding to an enzyme in its active site, reacting, then being released as products.
For many enzymes, the active site follows a lock and key (Figure \(\PageIndex{3A}\)) model where the substrate fits exactly into the active site. The enzyme and substrate must be a perfect match so the enzyme only functions as a catalyst for one reaction. Other enzymes have an induced fit (Figure\(\PageIndex{3B}\)) model. In an induced fit model, the active site can make minor adjustments to accommodate the substrate. This results in an enzyme that is capable of interacting with a small group of similar substrates. Look at the shape of the active site compared to the shape of the substrate in B of the figure below. The active site adjusts to accommodate the substrate.

Figure \(\PageIndex{3}\): (A) Lock and key enzyme model and (B) induced fit enzyme model.
Cofactors and Coenzymes
Some enzymes require the presence of another substrate as a "helper" molecule in order to function properly. Cofactors and coenzymes serve in this role. Cofactors are inorganic species and coenzymes are small organic molecules. Many vitamins, such as B vitamins, are coenzymes. Some metal ions which function as cofactors for various enzymes include zinc, magnesium, potassium, and iron.
Classes of Enzymes
Enzymes are named consisting of the root of the name of its substrate(s) with the -ase suffix and classified by the types of reaction they catalyze.
| Class | Type of Reaction Catalyzed | Examples |
|---|---|---|
| oxidoreductases | oxidation-reduction reactions | Dehydrogenases catalyze oxidation-reduction reactions involving hydrogen and reductases catalyze reactions in which a substrate is reduced. |
| transferases | transfer reactions of groups, such as methyl, amino, and acetyl | Transaminases catalyze the transfer of amino group, and kinases catalyze the transfer of a phosphate group. |
| hydrolases | hydrolysis reactions | Lipases catalyze the hydrolysis of lipids, and proteases catalyze the hydrolysis of proteins |
| lyases | reactions in which groups are removed without hydrolysis or addition of groups to a double bond | Decarboxylases catalyze the removal of carboxyl groups. |
| isomerases | reactions in which a compound is converted to its isomer | Isomerases may catalyze the conversion of an aldose to a ketose, and mutases catalyze reactions in which a functional group is transferred from one atom in a substrate to another. |
| ligases | reactions in which new bonds are formed between carbon and another atom; energy is required | Synthetases catalyze reactions in which two smaller molecules are linked to form a larger one. |
Example \(\PageIndex{1}\)
Identify the substrate catalyzed by each enzyme.
-
lactase
-
cellulase
-
peptidase
Solution
-
lactose
-
cellulose
-
peptides
Exercise \(\PageIndex{2}\)
Identify the substrate catalyzed by each enzyme.
-
lipase
-
amylase
-
maltase
Example \(\PageIndex{2}\)
Identify each type of enzyme.
-
decarboxylase
-
protease
-
transaminase
Solution
-
lyase
-
hydrolase
-
transferase
Exercise \(\PageIndex{2}\)
Identify each type of enzyme.
-
dehydrogenase
-
isomerase
-
lipase
Summary
An enzyme is a biological catalyst, a substance that increases the rate of a chemical reaction without being changed or consumed in the reaction. A systematic process is used to name and classify enzymes. A substrate binds to a specific region on an enzyme known as the active site, where the substrate can be converted to product. The substrate binds to the enzyme primarily through hydrogen bonding and other electrostatic interactions. The induced-fit model says that an enzyme can undergo a conformational change when binding a substrate. Enzymes exhibit varying degrees of substrate specificity.
Contributors and Attributions
Allison Soult, Ph.D. (Department of Chemistry, University of Kentucky)
- " Enzymes" by LibreTexts is licensed under CC BY-NC-SA .

