8.6: Enzyme Activity
- Page ID
- 342728
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Outcomes
- Describe how substrate concentration, pH, and temperature affect enzyme activity.
- Interpret graphs of reaction rate vs. reaction conditions.
The most important property of enzymes is the ability to increase the rates of reactions occurring in living organisms. Determining how fast an enzymatic reaction occurs is a measure of enzyme (or catalytic) activity. Because most enzymes are proteins, their activity is affected by factors that disrupt protein structure, as well as by factors that affect catalysts in general. These factors include concentration, pH of the surroundings, and temperature.
Concentration
As with most reactions, the concentration of the reactant(s) affects the reaction rate. This is also true in enzyme concentration. When either substrate or enzyme concentration is low, the rate of the reaction will be slower than where there are higher concentrations. The two species must interact for a reaction to occur and higher concentrations of one or both will result in more effective interactions between the two.
Concentration of Substrate
In the presence of a given amount of enzyme, the rate of an enzymatic reaction increases as the substrate concentration increases until a limiting rate is reached, after which further increase in the substrate concentration produces no significant change in the reaction rate (Figure \(\PageIndex{1a}\)). At this point, so much substrate is present that essentially all of the enzyme active sites have substrate bound to them. In other words, the enzyme molecules are saturated with substrate. The excess substrate molecules cannot react until the substrate already bound to the enzymes has reacted and been released (or been released without reacting). When the enzyme becomes saturated with substrate, it would operate at steady state, the condition in which an enzyme is operating at maximum activity.
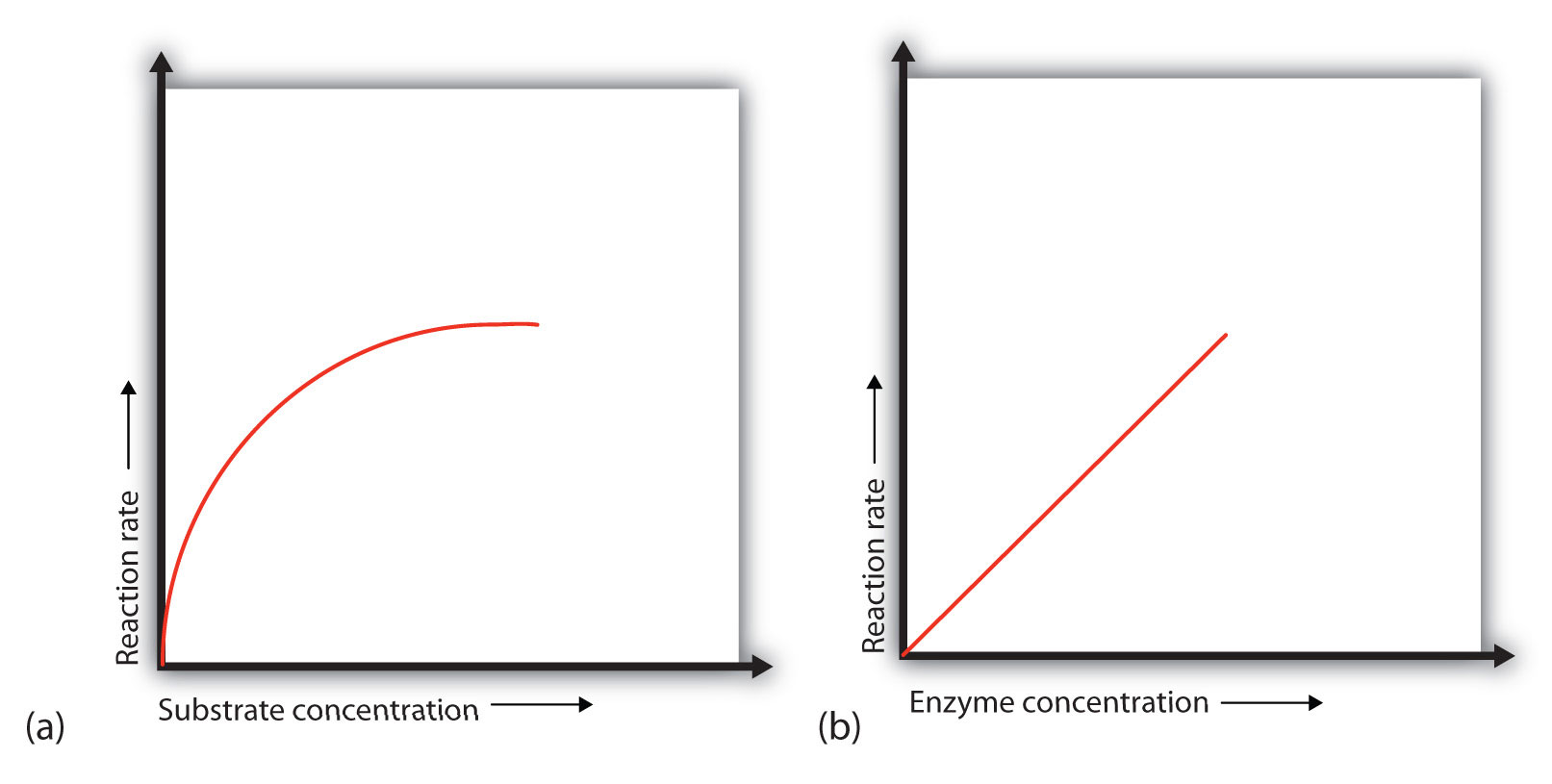
Let’s consider an analogy. Ten taxis (enzyme molecules) are waiting at a taxi stand to take people (substrate) on a 10-minute trip to a concert hall, one passenger at a time. If only 5 people are present at the stand, the rate of their arrival at the concert hall is 5 arrivals in 10 minutes. If the number of people at the stand is increased to 10, the rate increases to 10 arrivals in 10 minutes. With 20 people at the stand, the rate would still be 10 arrivals in 10 minutes. The taxis have been “saturated.” If the taxis could carry 2 or 3 passengers each, the same principle would apply. The rate would simply be higher (20 or 30 people in 10 minutes) before it leveled off.
Concentration of Enzyme
When the concentration of the enzyme is significantly lower than the concentration of the substrate (as when the number of taxis is far lower than the number of waiting passengers), the rate of an enzyme-catalyzed reaction is directly dependent on the enzyme concentration (Figure \(\PageIndex{1b}\)). This is true for any catalyst; the reaction rate increases as the concentration of the catalyst is increased.
pH
Some enzymes work best at acidic pHs, while others work best in neutral environments. For example, digestive enzymes secreted in the acidic environment (low pH) of the stomach help break down proteins into smaller molecules. The main digestive enzyme in the stomach is pepsin, which works best at a pH of about 1.5. These enzymes would not work optimally at other pH levels. Trypsin is another enzyme in the digestive system, which breaks protein chains in food into smaller particles. Trypsin works in the small intestine, which is not an acidic environment, and has an optimum pH is about 8.
Different reactions and different enzymes will achieve their maximum rate at certain pH values. An enzyme is most active at its optimum pH, which is the pH where it maintains the native tertiary structure. As shown in Figure \(\PageIndex{2}\), the enzyme achieves a maximum reaction rate at a pH of 4. Notice that the reaction will continue at lower and higher pH values because the enzyme will still function at other pH values but will not be as effective. At very high or very low pH values, denaturation will occur because an enzyme is just a protein with a specific function.

Figure \(\PageIndex{2}\): Relationship between rate and pH.
Temperature
As with pH, reactions also have an optimum temperature where the enzyme functions most effectively. It will still function at higher and lower temperatures, but the rate will be less. For many biological reactions, the optimum temperature is at physiological conditions which is around \(37^\text{o} \text{C}\) which is normal body temperature. Many enzymes lose function at lower and higher temperatures. At higher temperatures, an enzyme's shape deteriorates. Only when the temperature comes back to normal does the enzyme regain its shape and normal activity unless the temperature was so high that it caused irreversible damage.

Figure \(\PageIndex{3}\): Relationship between temperature and rate.
Example \(\PageIndex{1}\)
An enzyme has an optimum pH of 7.4. What is most likely to happen to the activity of the enzyme if the pH drops to 6.3? Explain.
Solution
The activity will decrease; a pH of 6.3 is more acidic than 7.4, and one or more key groups in the active site may bind a hydrogen ion, changing the charge on that group.
Exercise \(\PageIndex{1}\)
An enzyme has an optimum pH of 7.2. What is most likely to happen to the activity of the enzyme if the pH increases to 8.5? Explain.
Summary
Initially, an increase in substrate concentration leads to an increase in the rate of an enzyme-catalyzed reaction. As the enzyme molecules become saturated with substrate, this increase in reaction rate levels off. The rate of an enzyme-catalyzed reaction increases with an increase in the concentration of an enzyme. At low temperatures, an increase in temperature increases the rate of an enzyme-catalyzed reaction. At higher temperatures, the protein is denatured, and the rate of the reaction dramatically decreases. An enzyme has an optimum pH range in which it exhibits maximum activity.
Contributors and Attributions
Allison Soult, Ph.D. (Department of Chemistry, University of Kentucky)
- " Enzyme Activity" by LibreTexts is licensed under CC BY-NC-SA .

