Section 12.4: Biological Metal Storage
- Last updated
- Save as PDF
- Page ID
- 440907
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Iron
Iron is widely used in a wide number of biological applications. Ferrous (Fe2+) ion appears to have been the environmentally stable form during prebiotic times. The combination of the reactivity of ferrous ion and the relatively large amounts of iron used by cells may have necessitated the storage of ferrous ion; recent results suggest that ferrous ion may be stabilized inside ferritin long enough to be used in some types of cells. The ability of primitive organisms photosynthetically oxidize H2O into dioxygen, probably produced the worst case of environmental pollution in terrestrial history. As a result, the composition of the atmosphere, the course of biological evolution, and the oxidation state of environmental iron all changed profoundly. Paleontologists and meteorologists estimate that there was a lag of about 200 - 300 million years between the first dioxygen production and the appearance of significant dioxygen concentrations in the atmosphere, because the dioxygen produced at first was consumed by the oxidation of ferrous ions in the oceans. The transition in the atmosphere, which occurred about 2.5 billion years ago, caused the bioavailability of iron to plummet and the need for iron storage to increase. Comparison of the solubility of Fe3+ at physiological conditions (about 10-18 M) to the iron content of cells (equivalent to 10-5 to 10-8 M) emphasizes the difficulty of acquiring sufficient iron.
Iron is stored mainly in the ferritins, a family of proteins composed of a protein coat and an iron core of hydrous ferric oxide [Fe2O3(H2O)n] with various amounts of phosphate.6,7 As many as 4,500 iron atoms can be reversibly stored inside the protein coat in a complex that is soluble; iron concentrations equivalent to 0.25 M [about 1016-fold more concentrated than Fe(III) ions] can be easily achieved in vitro. Ferritin is found in animals, plants, and even in bacteria; the role of the stored iron varies, and includes intracellular use for Fe-proteins or mineralization, long-term iron storage for other cells, and detoxification of excess iron. Iron regulates the synthesis of ferritin, with large amounts of ferritin associated with iron excess, small or undetectable amounts associated with iron deficiency.
Ferritin
Despite the importance of iron for life, excess iron can be toxic.3 Unregulated free iron can catalyze the production of harmful reactive oxygen species (ROS) and free radicals, most commonly via Fenton chemistry. The Fenton reaction (below) is the reaction between iron(II) ions and hydrogen peroxide to produce the extremely reactive and harmful hydroxyl radical (•OH).1 ROS are generally strong oxidizing agents and can cause permanent cell damage, organ failure, and death
Fe2+ + H2O2 → Fe3+ + OH- + •OH
Structurally, ferritin is a hollow cage and the sequestered iron is stored as an iron(III) mineral within the protein shell (Figure \(\PageIndex{1}\)).2,3
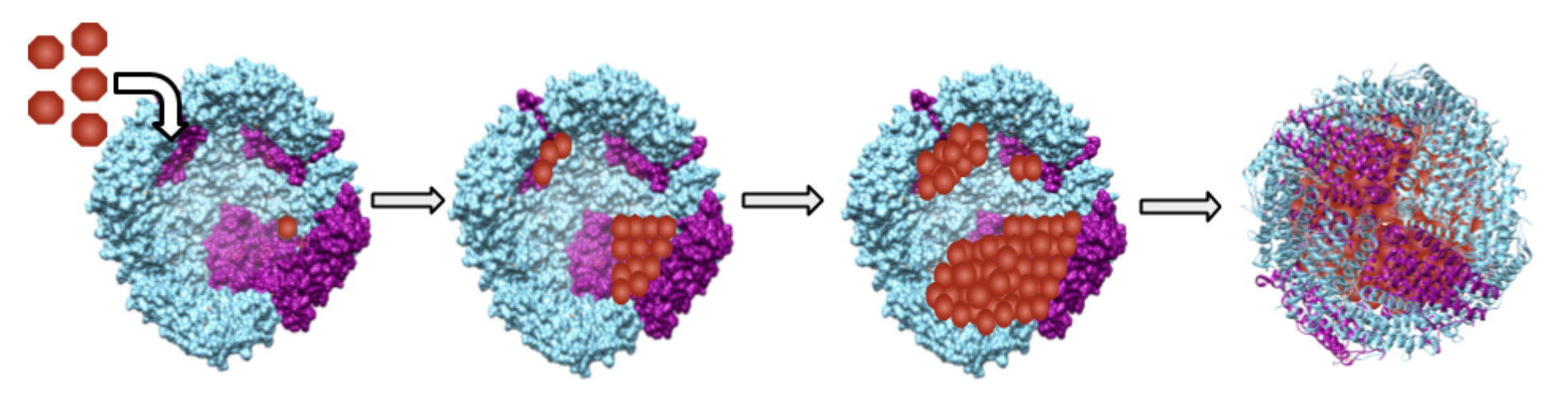
Figure \(\PageIndex{1}\): A cartoon diagram of sequestration of iron, which has been crystallized and is stored as an iron/oxy mineral in the hollow cage of ferritin. From left to right, the diagram depicts the cross section of the ferritin protein cage and the growing nucleation in the hollow center of ferritin. Each red shape represents some crystalline, mineral combination of iron, oxygen, and hydrogen (FeOOH core). Nucleation begins on the purple surfaces of ferritin, which will be discussed in detail later, but is otherwise random. The final image in the diagram on the far right is the spherical ribbon structure of full iron-containing ferritin protein with the cartoon depiction of iron/oxy mineral (red shapes) within the center. While this diagram depicts ferritin being filled to capacity with iron, in reality, ferritin can be filled as much or as little with iron, as needed. Thus, this figure can also be interpreted as different ferritin proteins in environments of different iron concentrations and so storing different amounts of iron.
Ferritin is made of subunits. Each subunit has four polypeptide α-helices or spirals.2 Structures of ferritin differ based on the organism which produces it. Most ferritin is made of 24 of these subunits.6 Human ferritin is a 24-subunit, 480 kDa, 12 nm globular protein with a hollow center cavity (cavity diameter of 8 nm).2 One 24-subunit ferritin protein can store up to 4500 iron ions in its hollow center.2 Ferritin is a very good space-saving model for the cell because of its high iron to protein ratio (4500 iron ions/8 nm cavity).2,9 This iron to protein ratio is 200 times that of hemoglobin.9 Up to 24% of ferritin’s weight could be stored iron ions. Meanwhile, bacterial ferritin can be either a 12- or 24-subunit globular protein.6 However, it is thought that the 12-subunit ferritin’s may play an additional role in protecting DNA from oxidative damage, while the 24-subunit bacterial ferritin may control bacterial iron metabolism.3
The subunits of human ferritin are classified as the heavy subunit or “H-chain” and the light subunit or “L-chain” (Figure \(\PageIndex{2}\)).6 The two subunits, however, are not extremely different in molecular weight or amino acid sequence. The H-chain is made of 178 amino acids with a molecular weight of 21 kDa and the L-chain is made 171 amino acids and weighs 18 kDa.4 The two types of subunits have ~53% protein sequence identity.4 The designations of heavy and light are actually a modern recasting of designations that originally reflected the organs from which the two forms were isolated, “H” for heart and “L” for liver.8
The two types of subunits combine in different ratios to form the 24-subunit protein shell in humans (Figure \(\PageIndex{2}\)).6 This ratio is dependent on the tissue where the ferritin is synthesized.6 The H subunit is responsible for catalyzing the oxidation of iron(II).8 The L subunit hosts the site of nucleation and storage of iron.8 The ratio of H:L will be higher in tissues where iron oxidation activity is high and iron detoxification is needed, such as the heart or the brain.1,4 Tissues, like those in the spleen, are used more for storage and will have a lower H:L ratio. The human liver produces ferritin that is 50% H and 50% L.
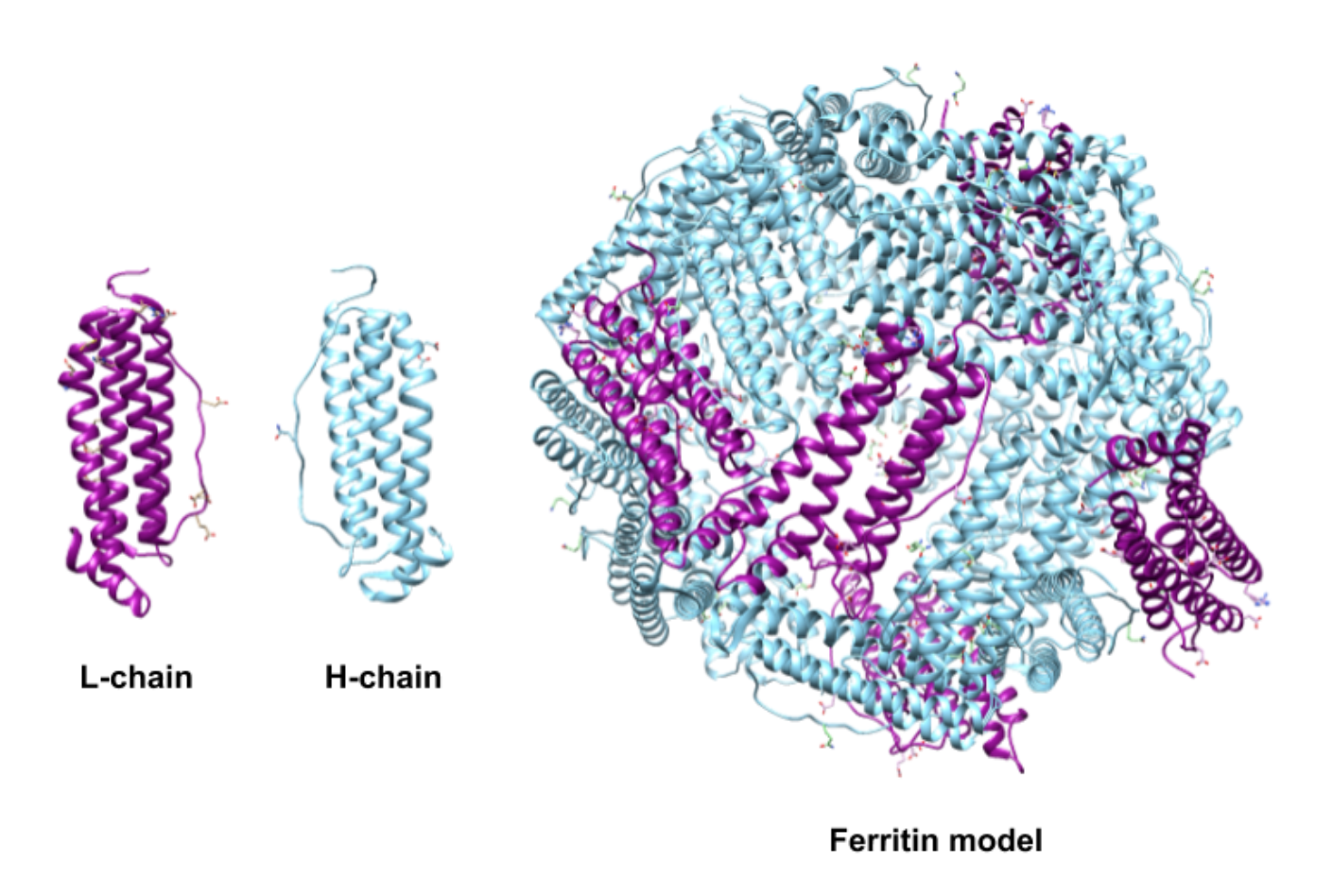
Figure \(\PageIndex{2}\): Light (L) (PDB 2FFX) and heavy (H) (PDB 2FHA) human ferritin subunit chains combine to form the human ferritin shell in specific ratios. The H:L ratio is dependent on the tissue where the protein is synthesized. The model seen here was created from the crystal structures of individual subunits, not a biologically obtained structure. However, it is approximately 30% L-type subunit and 70% H-type subunit and would be of a similar ratio to the ferritin found in the brain.1
Selectivity for Iron
Fe2+ ions are brought to ferritin for storage by the transport protein transferrin. The ions are believed to enter through one of the eight symmetric 3-fold channels or one of the six symmetric 4-fold channels formed by the subunits in the ferritin structure highlighted in Figure \(\PageIndex{3}\).6

Figure \(\PageIndex{3}\). Symmetric channels of entry and exit in Ferritin (PDB: 1FHA). The 3-fold channel on the left appears eight times in the 24 subunit ferritin protein. The 3-fold channel is formed by three subunits. The 4-fold channel on the right appears six times in the 24 subunit ferritin protein. The 4-fold channel is formed by four subunits.
The 3-fold channels are lined with the polar side chains aspartate and glutamate and make the channel hydrophilic.2 The hydrophilicity of the channel allows for the transport of water, metal cations, and hydrophilic molecules of an appropriate size into and and out of the ferritin center.4 Most studies indicate that the 3-fold channel is the main channel for Fe2+ ions both into and out of the cell.1,3 The 4-fold channels are lined with the non-polar side chain leucine and make the channel hydrophobic. It is widely thought that the 4-fold channels are involved with the diffusion of oxygen and hydrogen peroxide into and out of the ferritin center.1
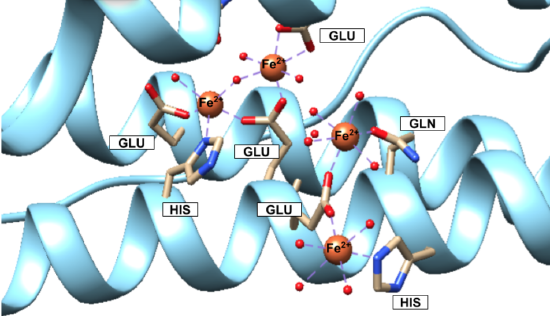
Iron(II) ions are oxidized at the “ferroxidase site” on the H-chain subunits (Figure \(\PageIndex{4}\)). After conversion to Fe3+, the iron is stored as a crystalline iron/oxy mineral.2 The mineral accumulates on the L-chain (Figure \(\PageIndex{5}\)).8
 |
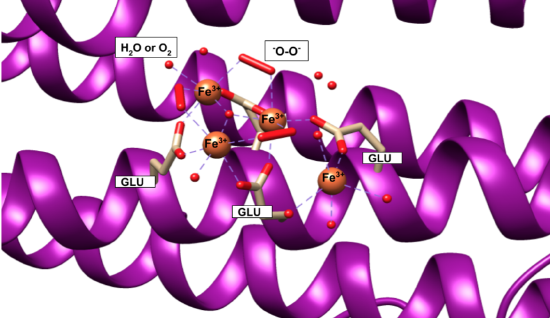 |
Figures 4 and 5 show iron(II) at the ferroxidase site and iron/oxy mineral at the nucleation site, according to the available crystal structures. The structure of the entire iron mineral core, however, is actually inconsistent throughout the whole mineral and differs even between identical ferritin shells. Traditionally, it was believed that the iron(III)/oxy mineral that makes up the core was similar to ferrihydrite and ideally structured as 20% tetrahedral and 80% octahedral; however, as more evidence has become available this model has been replaced by a “polyphasic” or heterogeneous model of the iron core that is thought to be more accurate.2,4 According to the polyphasic model, the mineral structure is heterogeneous in its chemical content and geometric structure.4 X-ray absorption fine structure (EXAFS) studies concluded that the iron core of ferritin was made of iron ions that are surrounded by six or seven oxygen atoms, surrounded by another shell of iron ions.2 This inconsistency leads to the conclusion that the mineral is a heterogeneous, hydrated iron(III)/oxy.3 In other words, the iron core itself is not packed regularly or in a pattern within ferritin.6
Selectivity of iron(II) by ferritin can be explained through hard-soft acid base (HSAB) theory. Ferritin binds an Fe2+ ion for oxidation. Fe2+ is categorized as a borderline acid. The binding sites on the H-chain are glutamic acid (Glu), histidine (His), and glutamine (Gln) residues (Figure \(\PageIndex{4}\)).6 Glu and Gln are categorized as hard bases, while His is categorized as borderline base. The oxidizing agents—oxygen molecules, hydrogen peroxide, or water molecules all have hard character. Fe2+ as a borderline acid binds well with the binding site of mixed hard and borderline character. The selection of Fe2+ in ferritin is further supported by experimental work where metals like zinc (which also exists in the body) were also shown to have binding abilities to ferritin.5 But, zinc, although it is also borderline, has softer character than Fe2+ and so would not be as stable or favorable with the hard Glu and Gln ligands.10
Iron Mineralization
Iron(II) is oxidized at the ferroxidase site on the H-chain (sites A and B) by some oxidizing agent, most probably O2 and H2O2.1 Three reactions/models for Fe2+ oxidation and mineralization have been observed. There is a ferroxidation reaction or protein catalysis model (Reactions \PageIndex {1} -\PageIndex {4} ), a mineralization reaction or crystal growth model (Reaction \PageIndex {5} ), and a detoxification reaction of Fe2+ + H2O2 (Reaction \PageIndex {6} ).1 The ferroxidation reaction occurs at the ferroxidase site on the H-chain of ferritin. The ferroxidation reaction for the protein catalysis model of ferritin oxidation can be written as:
\[ [2 Fe^{2+} + O_2 + 4 H_2O \rightarrow [Fe^{3+}\negthinspace-\negthinspace O\negthinspace -\negthinspace O\negthinspace -\negthinspace Fe^{3+}]^{2+}\]
\[Fe^{3+}\negthinspace -\negthinspace O\negthinspace -\negthinspace O\negthinspace -\negthinspace Fe^{3+}]^{2+} ] \rightarrow [Fe_2O\negthinspace -\negthinspace (OH)_2]^{2+}\]
\[ [Fe_2O\negthinspace -\negthinspace (OH)_2]^{2+} \rightarrow 2 FeOOH_{core} + H_2O_2 + 4 H^+ \]
\[\text {Net:}\ 2Fe^{2+} + O_2 + 4 H_2O \rightarrow 2FeOOH_{core} + H_2O_2 + 4 H^+ \]
The net mineralization reaction for the crystal growth model of ferritin oxidation is written as:
\[ 4 Fe^{2+} + O_2 + 6 H_2O \rightarrow 4 FeOOH_{core} + 8 H^+ \]
The most recently identified of the oxidation and mineralization reactions, the Fe2+ + H2O2 detoxification reaction is written as:
2 Fe2+ + H2O2 + 2 H2O → 2 FeOOHcore + 4 H+ (3.).1
Iron Release
When cells require iron, for enzyme synthesis, after blood loss, or during embryonic development, the iron stored in ferritin must be released rapidly, on demand, and under control.8 Although, as with many aspects of ferritin function, the mechanism of iron release to the cell from ferritin is unknown. What is known inherently about the process is that over its course it must essentially reverse mineralization: the iron(III)/oxy mineral must be dissolved from its solid state to aqueous ions and the iron(III) ions must be reduced to iron(II).
Proposed models for ferritin iron release include (1) an equilibrium between the iron stored in ferritin and the iron in the cytoplasm of the cell, (2) ferritin protein degradation, (3) spontaneous, direct dissolution of iron(III) from the mineral core from scavenging by iron(III) binding proteins, and (4) the reduction of the iron(III) mineral which is then complexed iron(II) by a chelating agent and transported out of ferritin.4,12 The last model (4) lacks confirmation, yet is considered by many to be the physiological mechanism. This model is the most efficient method and the presence of reducing agents under physiological conditions.4
Except the second model (2), the physiological relevance of all above models is still to be seen.12 The ferritin degradation model (2) is the only mechanism to have been observed under physiological conditions. However, since this model would require iron release to be dependent of the turnover and synthesis of ferritin, another model is suspected to be necessary.12
However iron is released from ferritin, studies indicate that the physical exit of the iron from ferritin takes place via the 3-fold channels. One proposal for exit based on observations in bacterial ferritin is the localized folding/unfolding of ferritin.4 The ferritin channels can be unfolded without affecting the overall function or structure of the protein. Unfolding the channels and thus, widening the opening, would allow for quick release of demineralized iron. The channels become highly disordered when this occurs to the effect that they do not appear in crystal structures.8
Much remains to be determined about human ferritin. Other functions of ferritin in the body, and the mechanisms for how Fe2+ enters and binds to ferritin, how Fe2+ is oxidized to Fe3+ for storage, and how iron is released remain undetermined, among others, are thought to be non-universal—even within the same organism.3,6
While much of the chemistry behind ferritin is, as of now, unexplained, ferritin is still an important protein for all life. Wherever iron is noted as an essential nutrient, ferritin must also be present for management and storage. The elucidation of ferritin can lead to advancements in iron metabolism and neurological disorders and new uses of ferritin chemistry in nanochemistry and catalytic industrial applications.3,4
Sources
[1] Bou-Abdallah, F. The Iron Redox and Hydrolysis Chemistry of the Ferritins. Biochimica et Biophysica Acta (BBA) - General Subjects 2010, 1800 (8), 719–731.
[2] Crabb, E.; Moore, E. Metals and life; Royal Society of chemistry: Cambridge, 2010.
[3] Theil, E. C.; Tosha, T.; Behera, R. K. Solving Biology’s Iron Chemistry Problem with Ferritin Protein Nanocages. Accounts of Chemical Research 2016, 49 (5), 784–791.
[4] Carmona, F.; Palacios, Ò.; Gálvez, N.; Cuesta, R.; Atrian, S.; Capdevila, M.; Domínguez-Vera, J. M. Ferritin Iron Uptake and Release in the Presence of Metals and Metalloproteins: Chemical Implications in the Brain. Coordination Chemistry Reviews 2013, 257 (19–20), 2752–2764.
[5] Knovich, M. A.; Storey, J. A.; Coffman, L. G.; Torti, S. V. Ferritin for the Clinician. Blood Rev 2009, 23 (3), 95–104.
[6] Bradley, J. M.; Le Brun, N. E.; Moore, G. R. Ferritins: Furnishing Proteins with Iron. JBIC Journal of Biological Inorganic Chemistry 2016, 21 (1), 13–28.
[7] Watt, G. D.; Jacobs, D.; Frankel, R. B. Redox Reactivity of Bacterial and Mammalian Ferritin: Is Reductant Entry into the Ferritin Interior a Necessary Step for Iron Release? Proceedings of the National Academy of Sciences 1988, 85 (20), 7457–7461.
[8] Bertini, I. Biological Inorganic Chemistry: Structure and Reactivity; University Science Books, 2007.
[9] Chasteen, N. D.; Harrison, P. M. Mineralization in Ferritin: An Efficient Means of Iron Storage. Journal of Structural Biology 1999, 126 (3), 182–194.
[10] Joshi, J. G.; Sczekan, S. R.; Fleming, J. T. Ferritin—A General Metal Detoxicant. Biol Trace Elem Res 1989, 21 (1), 105.
[11] Bertini, I.; Lalli, D.; Mangani, S.; Pozzi, C.; Rosa, C.; Theil, E. C.; Turano, P. Structural Insights into the Ferroxidase Site of Ferritins from Higher Eukaryotes. Journal of the American Chemical Society 2012, 134(14), 6169–6176.
[12] Honarmand Ebrahimi, K.; Hagedoorn, P.-L.; Hagen, W. R. Unity in the Biochemistry of the Iron-Storage Proteins Ferritin and Bacterioferritin. Chemical Reviews 2015, 115 (1), 295–326.
[13] H. Rodriguez, J.; Mccusker, J. Density Functional Theory of Spin-Coupled Models for Diiron-Oxo Proteins: Effects of Oxo and Hydroxo Bridging on Geometry, Electronic Structure, and Magnetism. Journal of Chemical Physics 2002, 116.
[14] Bertini, I., Bioinorganic Chemistry; Ed.; University Science Books: Mill Valley, Calif, 1994.
Contributed By
This work was originally written by Anna Shadid, Spring 2018: Anna is currently (as of 2018) a junior chemistry major at Saint Mary's College in Notre Dame, IN.
This work was originally edited by Dr. Kathryn Haas, Associate Professor, and Madison Sendzik, Teaching and Research Assistant, of Saint Mary's College.
Zinc, Copper, Vanadium, Chromium, Molybdenum, Cobalt, Nickel, and Manganese
Ions of nonferrous transition metals require a much less complex biological storage system, because the solubilities are much higher (≥ 10-8 M) than those for Fe3+. As a result, the storage of nonferrous transition metals is less obvious, and information is more limited. In addition, investigations are more difficult than for iron, because the amounts in biological systems are so small. Essentially nothing is known yet about the storage of vanadium, chromium, molybdenum, cobalt, nickel, and manganese, with the possible exception of accumulations of vanadium in the blood cells of tunicates.
Zinc and copper, which are used in the highest concentrations of any of the non-ferrous transition metals, are specifically bound by the protein metallothionein. (see Figure \(\PageIndex{8}\)). Like the ferritins, the metallothioneins are a family of proteins, widespread in nature and regulated by the metals they bind. In contrast to ferritin, the amounts of metal stored in metallothioneins are smaller (up to twelve atoms per molecule) and the amount of protein in cells is less. Because the cellular concentrations of the metallothioneins are relatively low and the amount of metal needed is relatively small, it has been difficult to study the biological fate of copper and zinc in living organisms, and to discover the natural role of metallothioneins. However, the regulation of metallothionein synthesis by metals, hormones, and growth factors attests to the biological importance of the proteins. The unusual metal environments of metallothioneins have attracted the attention of bioinorganic chemists.
Metallothioneins, especially in higher animals, are small proteins rich in cysteine (20 per molecule) and devoid of the aromatic amino acids phenylalanine and tyrosine. The cysteine residues are distributed throughout the peptide chain. However, in the native form of the protein, the peptide chains fold to produce two clusters of -SH, which bind either three or four atoms of zinc, cadmium, cobalt, mercury, lead, or nickel. Copper binding is distinct from zinc, with 12 sites per molecule.
In summary, iron is stored in iron cores of a complicated protein. Ferritin, composed of a hollow protein coat, iron-protein interface, and an inorganic core, overcomes the problems of redox and hydrolysis by directing the formation of the quasi-stable mineral hydrous ferric oxide inside the protein coat. The outer surface of the protein is generally hydrophilic, making the complex highly soluble; equivalent concentrations of iron are ≤ 0.25 M. By contrast to iron, storage of zinc, copper, chromium, manganese, vanadium, and molybdenum is relatively simple, because solubility is high and abundance is lower. Little is known about the molecules that store these metals, with the possible exception of metallothionein, which binds small clusters of zinc or copper.

