Section 12.3: Biological Dioxygen Transport and Storage
- Last updated
- Save as PDF
- Page ID
- 440906
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Oxygen Transport
Most organisms require molecular oxygen in order to survive. The oxygen is used in a host of biochemical transformations, although most is consumed in the reaction that is the terminal (or primary) step of oxidative phosphorylation to form ATP.
\[O_{2} + 4 H^{+} + 4 e^{-} \rightarrow 2H_{2}O \]
The transportation of oxygen from the air or water to the cells of an organism is vital. In the three chemically distinct dioxygen carrier proteins that have evolved and are found today, the dioxygen binding site in the protein, the active site, is an iron or copper complex. For hemoglobins, the most widely distributed family of dioxygen carriers, the active site has long been known to consist of an iron porphyrin (heme) group embedded in the protein. Almost all hemoglobins share the basic structure illustrated in Figure 4.2. Hemocyanin and hemerythrin, the other two biological dioxygen carriers, feature pairs of copper atoms and iron atoms, respectively, at the active sites.* Some basic properties of these metalloproteins are summarized in Table \(\PageIndex{1}\).
| Metalloprotein | Active Site of deoxy | Color Change deoxy → oxy | MW (Dalton) | # Subunits | Average MW Subunit (Dalton) |
|---|---|---|---|---|---|
| Hemoglobins | |||||
| Vertebrate | |||||
| Human A | heme FeII | purple → red | 64,000 | 4 | 16,000 |
| Invertebrate | |||||
| Erythrocruroin (Lumbricus terrestris, earthworm) | heme FeII | purple → red | up to 3.3 x 106 | 192 | 17,000 |
| Chlorocruorn (Eudistylia vancouveri, feather duster worm) | chloroheme FeII | purple → green | 3.1 x 106 | 192 | 15,000 |
| Hemocyanins | |||||
| Mollusk (Helix pomatia-\(\alpha\), edible snail) | Cu1 . . . Cu1 | colorless → blue | -9 x 106 | 160 | 52,700 |
| Arthropod (Cancer magister, crab) | Cu1 . . . Cu1 | colorless → blue | -9 x 105 | 12 | 76,600 |
| Hemerythrins (Phascolopsis syn. golfingia gouldii) | FeII . . . FeII | colorless → burgundy | 108,000 | 8 | 13,500 |
| * The use of the prefix hem- is confusing. In this context hem connotes blood. Thus, since hemocyanin and hemerythrin lack a heme group [an iron(II) porphyrin], they are nonheme metalloproteins. | |||||
In many organisms an additional dioxygen-binding protein, which stores dioxygen, is located in tissues that are subject to sudden and high dioxygen demand, such as muscles. These dioxygen-storage proteins are prefixed myo- (from the Greek root mys for muscle). Thus for the dioxygen-transport protein hemerythrin there exists a chemically similar dioxygen-storage protein myohemerythrin. For the hemoglobin family the corresponding storage protein is called myoglobin. Interestingly, some organisms that use hemocyanin as the dioxygen-transport protein use myoglobin as the dioxygen-storage protein.
Introduction to Myoglobin and Hemoglobin
Myoglobin: O2 storage
The respiratory system is an organ system in the body that functions in gas exchange with the environment. Exchange of gases like carbon dioxide (CO2) and dioxygen (O2) are essential for sustaining life forms. O2 is necessary in aerobic metabolism for oxidative phosphorylation (synthesis of ATP) at the electron transport chain (ETC).2 ATP is the energy source needed for muscular contraction in mammals. ATP synthesis requires oxygen as an electron acceptor in the ETC, therefore oxygen must be readily available for use in metabolically active muscles. Since muscles need large quantities of O2, it is transported by proteins in the blood and stored in muscle tissue. One of these proteins is myoglobin. Myoglobin is a hemoprotein found in the skeletal muscle of mammals that functions in oxygen storage and diffusion. The heme in myoglobin can reversibly bind a O2 molecule to regulate the transportation of O2 from red blood cells to mitochondria when skeletal muscles are metabolically active.
Structure of Myoglobin
The structure of myoglobin (Figure \(\PageIndex{2}\)) is similar to the structure of one of the \(\beta\) subunits of hemoglobin. Myoglobin and hemoglobin are both part of the globin family; a family of heme-containing globular polypeptides with eight \(\alpha\)-helices in their protein fold. Myoglobin contains only one subunit of globin, while hemoglobin has four subunits.
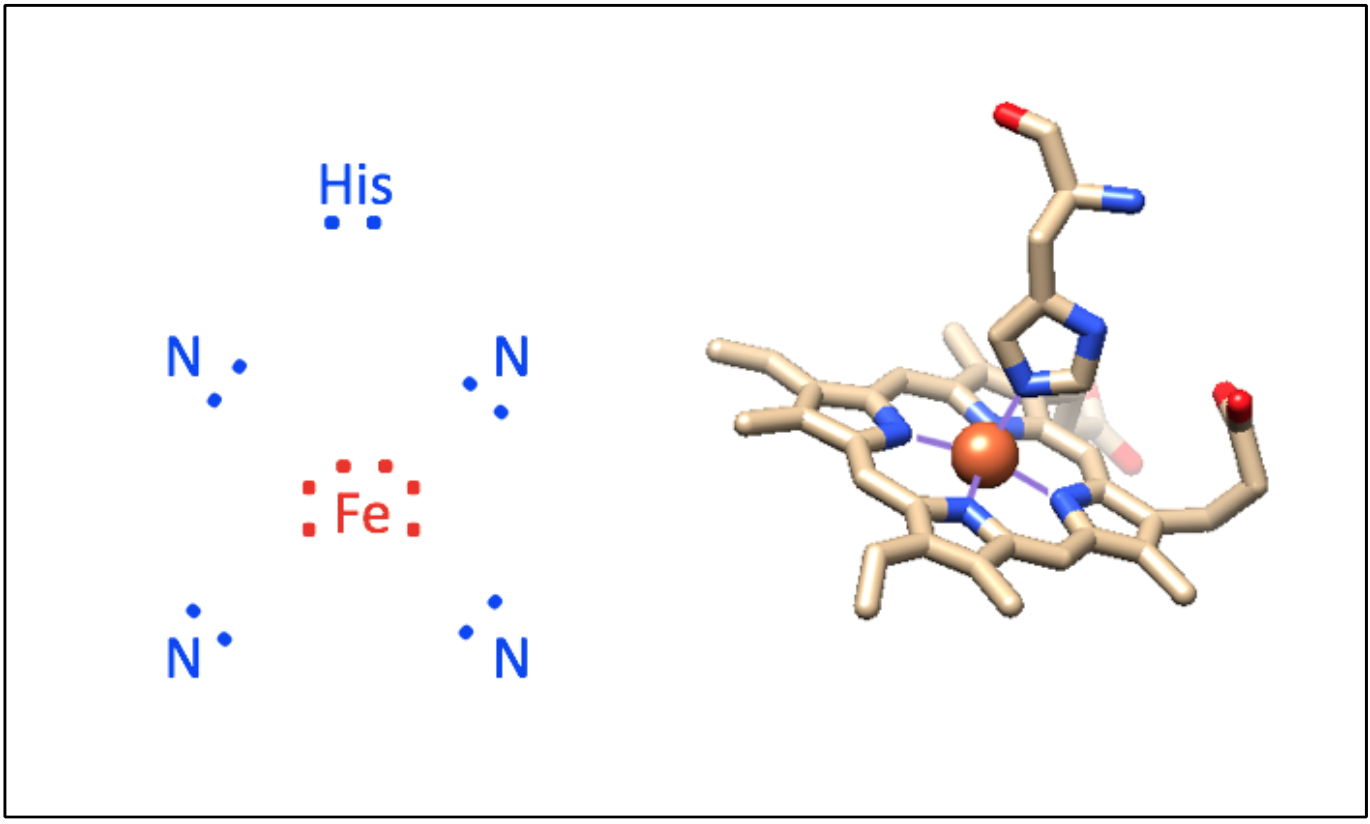
The iron-containing heme group allows myoglobin to reversibly bind to O2 (Figure \(\PageIndex{2}\)). Heme is a large, aromatic porphyrin ring with four pyrrole nitrogens bound to an Fe(II) ion at the center (Figure \(\PageIndex{2}C\)).2,3 The nitrogens from the porphyrin ring and a histidine serve as ligands for the Fe(II) metal center. The heme Fe is bound to the myoglobin polypeptide through the proximal histidine residue. The iron ion has six coordination sites: four equitorial sites are occupied by pyrole nitrogens of heme, and one axial site is occupied by a proximal histidine residue. The remaining axial coordination site is available for binding a O2 molecule (Figure \(\PageIndex{2}A-C\)).
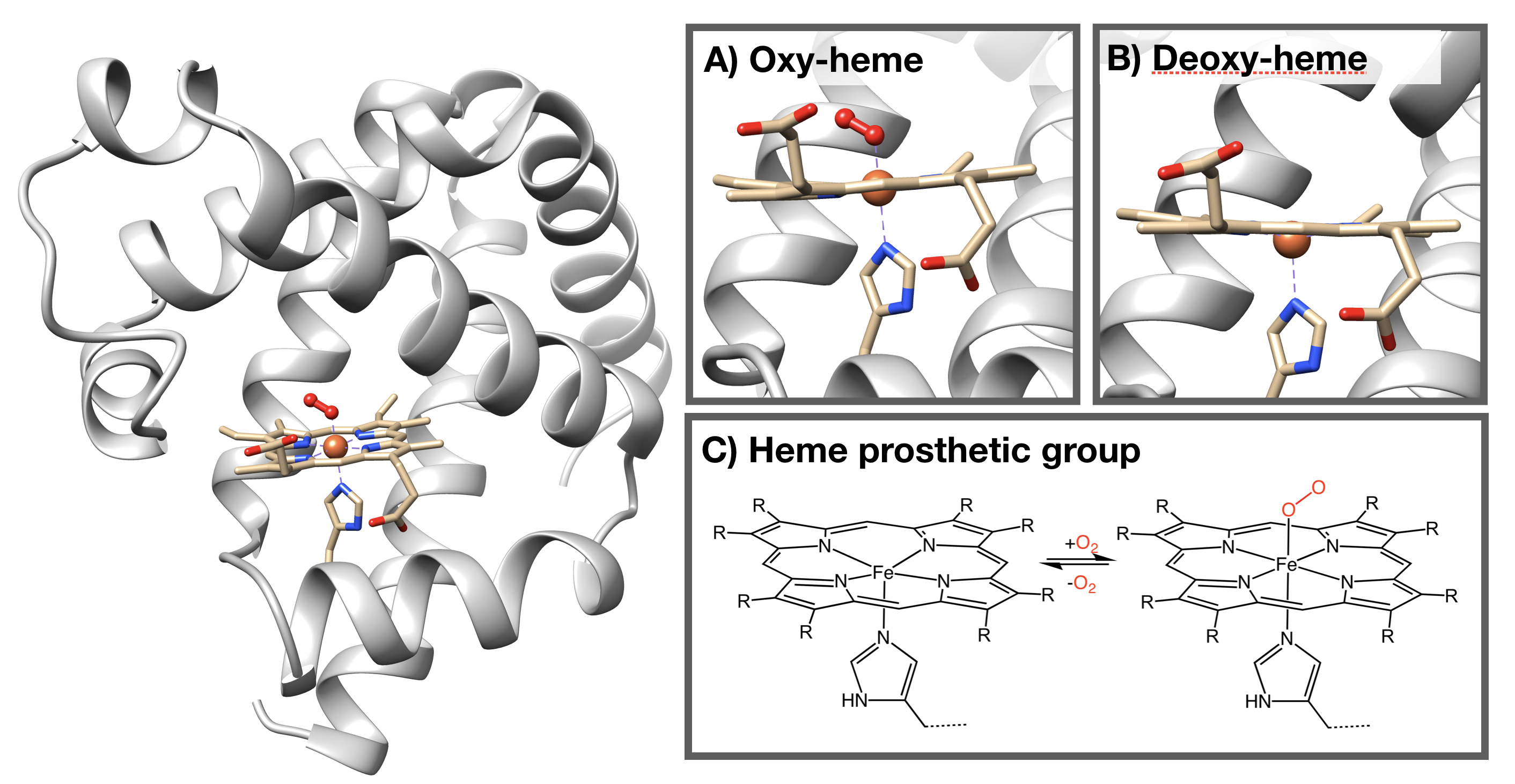
Reversible binding of oxygen
Now let’s move on to the function of myoglobin: oxygen storage. Myoglobin can reversibly bind a dioxygen molecule to regulate the transportation of oxygen from red blood cells to mitochondria when skeletal muscles are metabolically active. This binding occurs at the iron center of the heme group. Although the 18-electron rule was a rule developed for organometallic complexes and chemistry, we can apply it here to explain the O2-binding behavior of myoglobin. In deoxymyoglobin, the total valence electron count around Fe is 16 electrons (See Fig. \(\PageIndex{2}\), 10 electrons from ligands plus 6 electrons from Fe(II)). Under the 18-electron rule, a predictable step for a 16-electron species is ligand addition. The binding of O2 to Fe(II) is a ligand addition reaction. Once O2 binds to the Fe(II) in myoglobin, the new valence electron count around Fe is 18 electron. A predictable step for an 18-electron species is a ligand dissociation reaction, as is the reaction of O2 dissociation from the Fe center. The binding and dissociation of O2 makes sense based on the 18-electron rule.
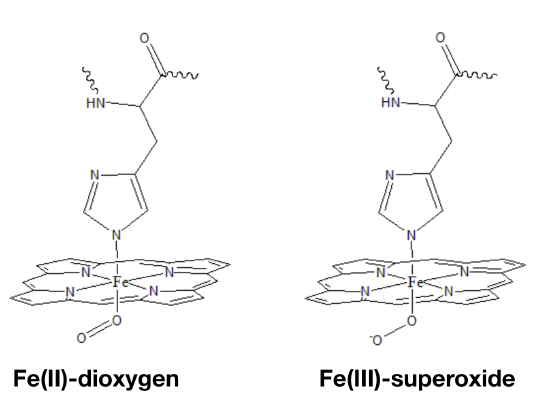
Iron oxidation state
The oxidation state of Fe in oxy-myoglobin is a highly debated topic because data is inconclusive and seemingly contradictory. Data from infrared (IR) spectroscopy and magnetic measurements have lead scientists to propose two different modes of oxygen binding to the iron ion. In one model (Figure \(\PageIndex{4}\) left), Fe(II) simply binds to O2. A second model predicts that electrons are transfered from the Fe center to the dioxygen molecule to create Fe(III) bound to superoxide (Figure \(\PageIndex{4}\) right). Although many debate whether the iron, after binding to oxygen, remains iron (II) or becomes iron (III), it is difficult to assign an oxidation state to the bound iron or the oxygen. The actual molecular orbitals that result from the iron-oxygen interaction have both iron and O2 character. It is therefore not quite correct to focus on the electrons "belong" to Fe or dioxygen. The two extremes, where one is that Fe becomes an iron superoxide by donating an electron from iron to the oxygen, and the other that Fe becomes an iron oxide by sharing the electrons are probably both incorrect. The molecular orbitals contain characteristics of both iron superoxide and iron oxide.
Sources
- Ordway, G. A. Journal of Experimental Biology 2004, 207(20), 3441–3446.
- Ahern, K.; Rajagopal, I.; Tan, T. Biochemistry Free For All; Creative Commons, 2017; Vol. 1.2.
- Casiday, R.; Frey, R. Hemoglobin and the Heme Group: Metal Complexes in the Blood for Oxygen Transport Inorganic Synthesis Experiment
- Penn State University. In Chem 324: Bio-Inorganic Chemistry.
- Lippard, S.J., Berg, J.M. Principles of Bioinorganic Chemistry. University Science Books. 1994. 284-289.
- Atkins, Overton, Rourke, Weller, Armstrong. Shriver and Atkins’ Inorganic Chemistry. Oxford University Press. 2010. 738-79.
- Richards, M. Antioxidants and Redox Signaling 2013. 2342-2351.
- Mazumdra, S., Medhi, O.K., Mitra, S. American Chemical Society. 1990. 700-705.
- Shriver, D., Atkins, P. Inorganic Chemistry, 5th ed.; W.H. Freeman and Company: New York, 2009; 739.
- Vanholder, R., Sever, M.S., Erek, E., Lameire, N. 2000. Rhabdomyolysis. Journal of the American Society of Nephrology 11:1553-1561
Hemoglobin
Hemoglobin, a polypeptide found in red blood cells, allows dioxygen (O2) to be transported within blood from the lungs to other tissues within the body. Hemoglobin is a polypeptide found in red blood cells. It allows for the transportation of O2 from the lungs to other tissues within the body. This molecule also is also responsible for the color of blood.1 Oxygenated blood is a bright red because hemoglobin absorbs green light of wavelengths 540-542 nm, and thus it results in a bright red colored solution.2 Deoxygenated blood, however, is darker in color because it absorbs a more yellow/green color of wavelength 554 nm, and thus produces a darker color of red.
Hemoglobin is able to transport oxygen within the body due to its unique structure. Its structure consists of four globin subunits: two α and two β subunits. Each subunit contains a heme prosthetic group with an iron bound (Figure 1). Hemoglobin exists in high concentrations in the cytoplasm of red blood cells, so it needs to be very soluble in aqueous cytoplasm. This requirement is reflected in the protein's globular shape, and the fact that it is folded in such a way that hydrophilic residues are found on the surface of the protein exposed to water, while hydrophobic residues are found on the interior of the protein. This folding enables hemoglobin to have a stable fold in aqueous solution that also allows the protein to interact favorably with water and to be soluble in the water-filled cytoplasm of the cell.

Cooperative binding of O2
An important feature of hemoglobin is a cooperative binding of oxygen to each subunit due to conformational changes upon oxygen binding to the heme iron. Hemoglobin exists in both the T-state (tense state) and the R-state (relaxed). The T-state has lower affinity for dioxygen due to the tilting of the proximal histidine and steric hindrance of the O2 coordination site. Steric hindrance makes it difficult for oxygen molecule to enter the site and bind to Fe. When an oxygen binds to one subunit of hemoglobin, the iron shifts into the plane of the porphoryn ring, and tugs on the proximal histidine.3,9 This causes the proximal histidine ring to be pulled toward the plane of the prosthetic group, decreasing the tilt of the histidine, causing a shift in the tertiary structure of that subunit, and displacing residues that were providing steric hiderance of the oxygen binding site. These conformational changes in one subunit cause similar changes to the tertiary structures of adjacent subunits, in turn decreasing steric and electrostatic constraints in those adjacent units. The result is adoption of the R-state and a subsequent increase in oxygen affinity to the other subunits.
When the iron ion is bound to only five coordination sites the iron (II) lies 0.4 Å outside of the porphyrin ring.3,9,12 When the oxygen binds to the iron core the iron becomes smaller as it becomes low-spin because electrons are pulled closer to the Fe core or are transfered to the dioxygen molecule due to backbonding. The low spin iron and is able to fit in the plane of the porphyrin ring.3,9
The short video below illustrates conformational differences between fully oxygenated hemoglobin and deoxygenated hemoglobin. (Click here if the video does not load).
References
- Casiday, R.; Frey, R. Washington University of St. Lous Chemistry. http://www.chemistry.wustl.edu/~edud...exinBlood.html (accessed 6 March).
- Zijistra, W. G; Buursma, A.; Meeuwsen-van der Roest, W. P. Absorption Spectra of Human Fetal and Adult Oxyhemoglobin, De-Oxyhemoglobin, Carboxyhemoglobin, and Methemoglobin. Clin Chem [online] 1991, 37, 1633-1638.
- Prahl, S. Oregon Medical Laser Center. https://omlc.org/spectra/hemoglobin/...uct/index.html (accessed 6 March).
- Berg, J. M.; Tymoczko, J. L.; Stryer, L. Biochemistry, 5 ed., W. H Freeman and Company: New York, 2002; unknown.
- Jensen, K. P.; Ryde, U.; How O2 Binds to Heme: Reasons for Rapid Binding and Spin
Inversion. JBC [online] 2004, 279, 14561-14569
- http://chemed.chem.purdue.edu/genchem/topicreview/bp/1biochem/blood3.html iron (III) oxide also talks about cooperativity
- http://nptel.ac.in/courses/104103069/module7/lec3/2.html iron (III) superoxide
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3230298/ Explains the oxygen dissociation curve
- Shriver, D; Atkins, P. Inorganic Chemistry, 5th ed.; W.H. Freeman and Company: New York, 2009; 739.
- Frausto da Silva J. J. R.; Williams R. J. P. The Biological Chemistry of the Elements: The Inorganic Chemistry of Life, 2nd ed.; Oxford University Press: Oxford, 2001; 370-373, 385-386.
- Crabb, E.; Moore, E., Eds. Metals and Life; Royal Society of Chemistry: United Kingdom, 2010.
- https://pubs.acs.org/doi/pdf/10.1021/cr00046a002 Low spin/high spin and cooperativity
- Spin-Pairing Model of Dioxygen Binding and Its Application to Various Transition-Metal Systems as well as Hemoglobin Coopera
Hemocyanin
Hemocyanin is the oxygen transporter protein present in the hemolymph of arthropods and mollusks.1 Hemolymph is the circulating fluid for these animals that is the invertebrate equivalent to vertebrate blood.2 In hemocyanin, the active site contains two copper ions. Hemocyanin is a type-3 copper protein, meaning that it contains of two copper centers, each coordinated by three histidine residues, as seen in Figure \(\PageIndex{6}\) and Figure \(\PageIndex{7}\). When deoxygenated (see Figure \(\PageIndex{6}\), left), the copper exists in the colorless, reduced Cu(I) state. Molecular oxygen binds at the copper site and is reduced to peroxide (\(\ce{O_{2}^{2-}}\)), Cu(I) is oxidized to Cu(II), and a blue complex forms. This change in the oxidation state of copper is responsible for the blue color of oxygenated invertebrate hemolymph. The oxygen bound as peroxide bridges the two copper centers together in μ-η2-η2 fashion.5
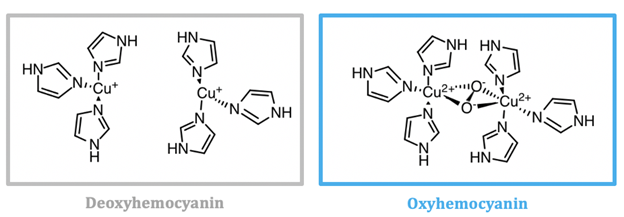
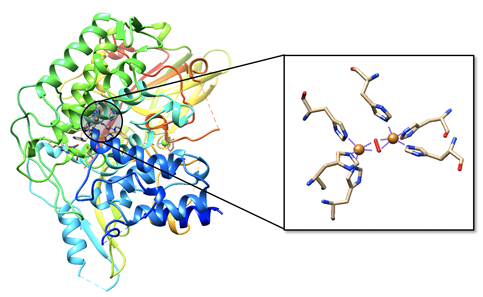
Mollusk and arthropod hemocyanins are structurally very different, however the active sites both contain two coppers at the center coordinated to three histidines. Arthropod hemocyanins have kidney-shaped subunits, each with an oxygen binding site, arranged into hexamers. Mollusk hemocyanin, on the other hand, is composed of about 10 subunits forming a hollow cylinder. Recently, there has been research into different therapeutic applications of hemocyanins. Studies suggest these metalloproteins have applications as viral and bacterial antigens, immune-stimulants for treatment of some cancers such as melanoma, and carrier molecules for vaccines.9
Note

The hemolymph of horeshoe crabs is incredibly valuable to pharmaceutical companies and biomedical researchers. It is the only known source of limulus amebocyte lysate, a substance which coagulates in the presence of bacterial endotoxin. This hemolymph extract is used to test medical devices, injectable drugs, and vaccines for bacterial contamination prior to distribution.
Learn more in this Chemistry World podcast
Image credit: CC BY; Amanda via Flickr
Structure
As seen in Figure \(\PageIndex{6}\) the active site of hemocyanin contains two copper ions, each bound to three histidine (His) residues. When the Cu(I) ions bind to oxygen they are oxidized to Cu(II). Cu(I) is a soft acid, Cu(II) is a borderline acid, and the imidazole ring of histidine is a borderline base. Because the copper ion alternates between a soft acid (Cu(I)) and a borderline acid (Cu(II)), it makes sense that it is bound to a borderline base. Borderline histidine is a better match for borderline Cu(II) than soft Cu(I), however His is still a better match for Cu(I) than a set of ligands that is harder. It is important to recognize, however, that there are other factors that affect metal ion selectivity, such as ionic size and ligand field stabilization energy, that must also be considered.
Hemocyanin exists in the hemolymph of invertebrates. Hemolymph is simply the invertebrate equivalent of blood that is present in vertebrates and is composed of water and organic and inorganic compounds. The inorganic metals present in hemolymph are sodium, chloride, calcium, magnesium, potassium, and manganese ions.11,12 \(\ce{Na^{+}}\), \(\ce{Ca^{2+}}\), \(\ce{Mg^{2+}}\), \(\ce{K^{+}}\), and \(\ce{Mn^{2+}}\) can all be seen as potential competitors for the copper at the active site due to the fact that they are all positively charged metal ions. They are all hard acids, indicating that they will likely not bind well with histidine, a borderline base. Based solely on HSAB, the histinines will prefer to bind to Cu(I)/Cu(II) because they are either soft/borderline acids. In addition to HSAB theory, it is also important to consider ionic size when determining if these competitors will bind in the site. These metal ions are larger than the copper ions, which may affect their ability to bind to the histidines in the pre-organized hemocyanin active site; the larger ions will not fit well into the pre-organized site, or if the site could accommodate the larger ion, it may change the protein structure to cause unfavorable steric interactions.
Kinetics and Thermodynamics
The stability of the complex is influenced by the energy of electrons in the d-orbital, as well as the geometry of the metal complexes. Because Cu(I) and Cu(II) have electrons at a higher energy, it makes them less stable and more likely to react. Additionally, Cu(II) is coordinated in a distorted tetrahedral geometry, but based on LFT, the square planar geometry is preferred. If Cu(II) was in the preferred geometry it would be more stable, and therefore reduction to Cu(I) and release of oxygen would be less favorable. Because Cu(II) is not fully stable in this environment, it allows for the cycling between the +1 and +2 oxidation states, and the transport of oxygen.
Lability is a desirable property for oxygen transport and storage proteins. The coordinated oxygen must be able to bind and unbind quickly and reversibly (carbon monoxide and cyanide gas are toxic because they will bind irreversibly with hemoglobin and displace oxygen). Cu(I) is a d10 metal and Cu(II) is a d9 metal, so their higher-energy antibonding t2 orbitals are occupied by electrons. When electrons occupy this higher-energy set of d-orbitals, the complex has a smaller CFSE and is more kinetically labile (has fast ligand substitutions reactions).
Redox Chemistry
The copper ions at the active site of hemocyanin cycle between Cu(I) and Cu(II) depending on whether or not oxygen is bound. Cu(I) is oxidized by oxygen when O2 coordinates with the metal. For the copper oxidation half reaction, the reduction potential at pH = 7 is 0.153 V.19 The one electron reduction of O2 is difficult because it is spin forbidden.20 The reduction potential of this reaction at pH = 7 is -0.33 V, which would make Erxn for the one electron redox reaction -0.48 V, a non-spontaneous reaction. The two electron oxidation of O2 to form H2O2 is much more favorable; the reduction potential of this reaction at pH = 7 is 0.281 V. This makes the overall which makes the overall Erxn 0.128 V, which makes the binding of oxygen to hemocyanin spontaneous (Figure \(\PageIndex{8}\)).
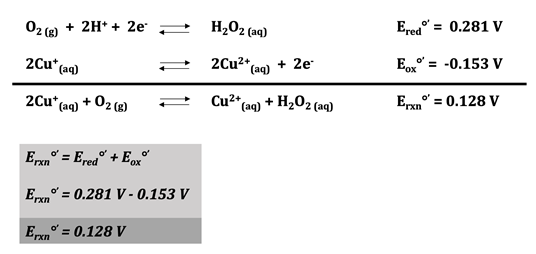
Figure \(\PageIndex{8}\):19 Oxidation-Reduction Reactions for Hemocyanin. The reduction half reaction can be seen on the first line. There are two electrons and two hydrogen ions involved in the reduction of oxygen, and the reduction potential of this reaction is 0.281 V at pH 7. On the following line, the oxidation half reaction can be seen where Cu(I) is oxidized to Cu(II) with an oxidation potential of -0.153 V at pH 7. The overall reaction appears on the third line. At pH 7, the redox potential of this reaction is 0.128 V.
Sources
- Cook, J. D.; Penner‐Hahn, J. E.; Stemmler, T. L. Structure and Dynamics of Metalloproteins in Live Cells. Methods in Cell Biology Methods in Nano Cell Biology 2008, 199–216.
- Kanost, M. R. Hemolymph. Encyclopedia of Insects 2009, 446–449.
- Christian, W. S.; Stefan, R. Functional morphology and diversity, Chapter 14: Circulatory System and Respiration; Oxford University Press: Oxford, 2013.
- Hashim, O. H.; Adnan, N. A. Coenzyme, Cofactor and Prosthetic Group — Ambiguous Biochemical Jargon. Biochemical Education 1994, 22 (2), 93–94.
- Kitajima, N.; Fujisawa, K.; Fujimoto, C.; Morooka, Y.; Hashimoto, S.; Kitagawa, T.; Toriumi, K.; Tatsumi, K.; Nakamura, A. A New Model for Dioxygen Binding in Hemocyanin. Synthesis, Characterization, and Molecular Structure of the .Mu.-.Eta.2:.Eta.2 Peroxo Dinuclear Copper(II) Complexes, [Cu(HB(3,5-R2pz)3)]2(O2) (R = Isopropyl and Ph). Journal of the American Chemical Society 1992, 114 (4), 1277–1291.
- IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). Online version (2019-) created by S. J. Chalk. ISBN 0-9678550-9-8. https://doi.org/10.1351/goldbook.
- IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). Online version (2019-) created by S. J. Chalk. ISBN 0-9678550-9-8. https://doi.org/10.1351/goldbook.
- Hazes, B.; Hol, W. Oxygenated Hemocyanin (Subunit Type Ii). 1996.
- Coates, C. J.; Decker, H. Immunological Properties of Oxygen-Transport Proteins: Hemoglobin, Hemocyanin and Hemerythrin. Cellular and Molecular Life Sciences 2016, 74 (2), 293–317.
- Libretexts. 3.2.1: Hard and Soft Acid and Base Theory. https://chem.libretexts.org/Courses/Saint_Mary's_College,_Notre_Dame,_IN/CHEM_342:_Bio-inorganic_Chemistry/Readings/Week_3:_Metal-Ligand_Interactions_continued..../3.2:_The_identity_of_metal_ion_and_the_ligand_donor_atom(s)_affects_affinity/3.2.1:_Hard_and_Soft_Acid_and_Base_Theory (accessed Jan 28, 2020).
- Pratoomchat, B.; Sawangwong, P.; Pakkong, P.; Machado, J. Organic and Inorganic Compound Variations in Haemolymph, Epidermal Tissue and Cuticle over the Molt Cycle in Scylla Serrata ž Decapoda/. 2002, 13.
- Sowers, A. D.; Young, S. P.; Grosell, M.; Browdy, C. L.; Tomasso, J. R. Hemolymph Osmolality and Cation Concentrations in Litopenaeus Vannamei during Exposure to Artificial Sea Salt or a Mixed-Ion Solution: Relationship to Potassium Flux. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 2006, 145 (2), 176–180. https://doi.org/10.1016/j.cbpa.2006.06.008.
- Morris, R. H.; Lee, A.; Hadzovic, A. A Tour of Hemocyanin. https://sites.chem.utoronto.ca/chemistry/coursenotes/GTM/JM/hemocyanin/start.htm (accessed Mar 27, 2020).
- Cirera, J.; Ruiz, E.; Alvarez, S. Stereochemistry and Spin State in Four-Coordinate Transition Metal Compounds. Inorganic Chemistry 2008, 47 (7), 2871–2889.
- Sundberg, R. J.; Martin, R. B. Interactions of Histidine and Other Imidazole Derivatives with Transition Metal Ions in Chemical and Biological Systems. Chemical Reviews 1974, 74 (4), 471–517.
- Nickerson, K. W.; Holde, K. E. V. A Comparison of Molluscan and Arthropod Hemocyanin—I. Circular Dichroism and Absorption Spectra. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry 1971, 39 (4), 855–872.
- Frieden, E.; Osaki, S.; Kobayashi, H. Copper Proteins And Oxygen: Correlations Between Structure And Function Of The Copper Oxidases. Journal of General Physiology 1965.
- Tommerdahl, A. P.; Burnett, K. G.; Burnett, L. E. Respiratory Properties of Hemocyanin From Wild and Aquacultured Penaeid Shrimp and the Effects of Chronic Exposure to Hypoxia. The Biological Bulletin 2015, 228 (3), 242–252.
- Lippard, S. J.; Berg, J. M. Principles of bioinorganic chemistry; University Science Books: Mill Valley, CA, 1994.
- Solomon, E. I.; Heppner, D. E.; Johnston, E. M.; Ginsbach, J. W.; Cirera, J.; Qayyum, M.; Kieber-Emmons, M. T.; Kjaergaard, C. H.; Hadt, R. G.; Tian, L. Copper Active Sites in Biology. Chemical Reviews 2014, 114 (7), 3659–3853.
Contributed By:
This work was originally written by:
- Lydia Lorenc, Spring 2018: Lydia is currently (as of 2018) a junior chemistry major at Saint Mary's College in Notre Dame, IN.
- Margaret Benjamin, a chemistry major at Saint Mary's College, class of 2020.
This work was originally edited by Dr. Kathryn Haas, Assistant Professor and Madison Sendzik, Teaching and Research Assistant of Saint Mary's College.

