16.10: Water
- Page ID
- 434976
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Relate the structure of water to some of its unique properties.
- Describe the different sources of fresh water available for use.
- Identify the different ions that can be present in water.
- Describe what makes water hard and how that hardness can affect its used.
Water composes the hydrosphere, which is described in Section 16.2. This section enlarges upon that discussion and the crucial role of water in the environment. Here the term natural water is used in reference to water that occurs in the environment in comparison to water in the anthrosphere, such as water in municipal water distribution systems. Water has a special place in living organisms and the environment. The quality and availability of water are of the utmost importance to humans and the environment. Although scarce and badly polluted in many parts of the world, water is arguably the most recyclable of the substances that compose Earth’s green capital and it is accurately described as the ultimate green substance.
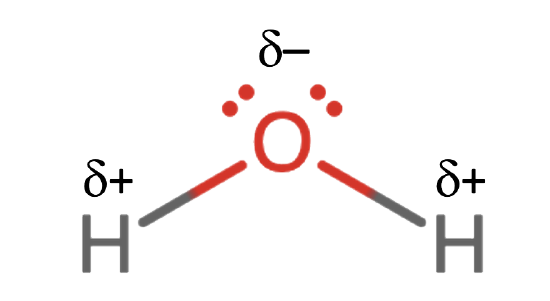
The chemical formula of water, H2O, is probably the best known of all compounds. This simple formula represents a substance that is unique and complex in its behavior. These special properties are due to the molecular structure of the H2O molecule described in previous chapters of this book and represented in Figure \(\PageIndex{1}\). There are two bonds between the O and H atoms and two sets of lone pairs on the O. The distribution of these lone pairs and bonds as far apart as possible around the O atom results in the two H-O bonds being located at an angle rather than a straight line. The side of the molecule with the two H atoms has a partial positive charge and the side with the two non-bonding pairs has a partial negative charge, so the molecule is polar. This polarity and the ability of the H atoms on one molecule to form hydrogen bonds to O atoms on other molecules determine the remarkable chemical and physical diversity of water.
|
|
|
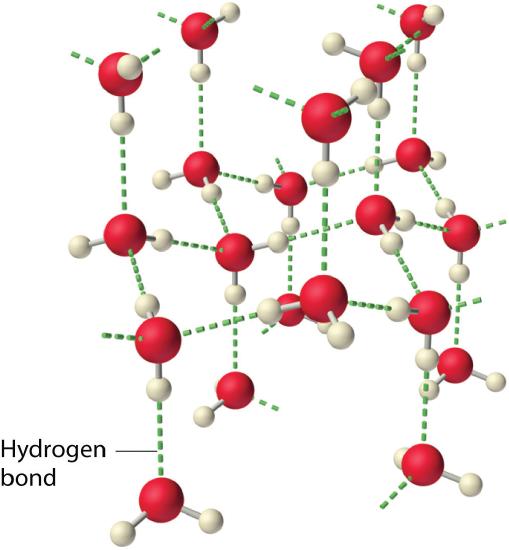 |
Especially because of their hydrogen bonding capability, water molecules are strongly attracted to each other. This means that a large amount of heat energy must be added to a mass of water to enable the molecules to move more rapidly as the temperature is raised. This gives water a very high heat capacity. A very large amount of energy must be added to a mass of ice to break the hydrogen bonds holding the molecules in place in the solid as it melts and an equally large amount of heat energy is released when liquid water freezes. Thus water has a very high latent heat of fusion. Even more energy per unit mass is required to convert liquid water to vapor (steam) and an equal amount of energy is released when water vapor condenses to liquid. This means that water has a very high heat of vaporization.
The ability of water to absorb, release, and store heat is crucial to its role in the environment and its practical uses. Water’s high heat capacity stabilizes temperatures of organisms and geographical areas. Steam produced in a boiler can be transferred through insulated pipes to remote locations and condensed to release heat. The heat released when atmospheric water condenses warms the surrounding air and is the driving force behind tropical storms. Europe owes its relatively mild weather, despite its northern latitudes, to heat carried by water across the North Atlantic Ocean from the Gulf of Mexico. As the water releases heat and cools along the European coasts, its density increases and it flows at lower ocean depths back to the Gulf of Mexico to repeat the cycle. Water’s high latent heat of fusion stabilizes temperatures of bodies of water at water’s freezing point (0 ̊ C).
In addition to those listed above, there are other unique and environmentally important qualities of water. It is an excellent solvent, especially for ionic substances, making it important in the transport of nutrients and wastes in the biosphere and in the dissolution, transport, and deposition of minerals in the geosphere. Water has a very high surface tension, a controlling factor in physiology and a property involved in formation of drops in rainfall. The fact that the maximum density of water occurs as a liquid at 4 ̊ C means that solid ice floats. If that were not the case, bodies of water in northern climates would become frozen solid with only a surface layer thawing during the summer. Water is largely transparent to visible light, which can penetrate the liquid to some depth and enable photosynthetic phytoplankton and some bottom-rooted plants in shallow water regions to carry out photosynthesis and produce the biomass that is the basis of aquatic food chains.
The Hydrosphere
The total water system surrounding the planet Earth is called the hydrosphere. It includes freshwater systems, oceans, atmosphere vapor, and biological waters. The Arctic, Atlantic, Indian, and Pacific oceans cover 71% of the Earth surface, and contain 97% of all water. Less than 1% is fresh water, and 2-3 % is ice caps and glaciers. The Antarctic Ice Sheet is almost the size of North America continent. These waters dominate our weather and climate, directly and indirectly affecting our daily lives. They cover 3.35x108 km2. The four oceans have a total volume of 1.35x109 km3.
Of the approximately 33 million km3 of freshwater in the hydrosphere, 86.9% is in the solid form in snowpack, ice and glaciers; 12.0% is accessible water under ground (groundwater), 0.37% is in freshwater lakes, reservoirs, and ponds; 0.31% is in saline lakes; 0.19% is soil moisture; 0.19% is in biospheric organisms; 0.039% is in the atmosphere as vapor and cloud droplets; 0.011% is in wetlands; and only 0.0051% constitutes the water in all Earth’s rivers, streams, and canals. Therefore, a remarkably small percentage of Earth’s water is relatively available for use, a major factor in the sustainability of this crucial part of Earth’s natural capital.
Groundwater
A body of rock which contains appreciable quantities of water is called an aquifer. Below the water table, the aquifer is filled (or saturated) with water. Above the water table is the unsaturated zone. Some regions have two or more water tables. These zones are usually separated by water-impermeable material such as boulder and clay. Groundwater, or the water in the rocks, can be brought to the surface by drilling below the water table and pumped out. The amount of water that can be pumped out depends on the structure of the aquifer. Little water is stored in tight granite layers, but large quantities of water are stored in limestone aquifer layers. In some areas, there are underground rivers.
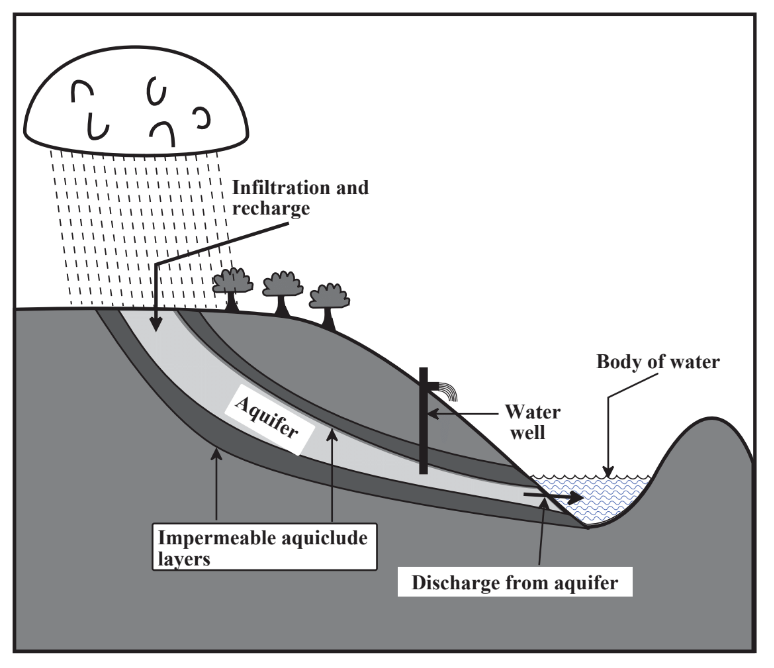
Figure \(\PageIndex{2}\) illustrates the pathway of groundwater into aquifers from precipitation falling on a watershed and infiltrating the aquifer through a recharge zone. The zone of saturation consists of the geospheric rock and soil layers in which all the pores are filled with water, the top of which defines the water table. The fraction of the aquifer formation consisting of pores is the porosity and the ability of the formation to allow movement of water is its permeability. Generally, high permeability and high porosity are desirable properties of aquifer formations that allow relatively large amounts of water to be withdrawn through water wells drilled into the aquifers. The quality of groundwater is strongly affected by its contact with mineral formations in the geosphere. Infiltration through rock and sand purifies water by filtering out microorganisms and suspended solids. Contact with geospheric solids largely determines the chemical composition of water by adding desirable levels of dissolved Ca2+ ion and alkalinity and sometimes undesirable solutes such as sodium chloride.
Surface Streams and Standing Bodies of Water
A major source of water is surface water that flows in streams and rivers. The areas of land upon which precipitation falls to provide flows of surface water is called the watershed. Insofar as water utilization is concerned, watersheds constitute one of the most important connections between the geosphere and the hydrosphere; the nature of the watershed largely determines the quantity and quality of water available for human use. A good watershed retains water for a significant length of time, which reduces flooding, allows for a steady flow of runoff water, and maximizes recharge of water into groundwater reservoirs (aquifer recharge, Figure \(\PageIndex{2}\)). Measures employed to enhance watershed quality include minimization of cultivation and forest cutting, especially on steeply sloping sections of watershed, construction of terraces and waterways planted with grass on cultivated land to minimize erosion and accumulation of sediment, construction of small impoundments on feeder streams of the watershed, and of course minimization of pollution such as from herbicides.
An important aspect of streams that affects water utilization and quality is their ability to mobilize sedimentary materials through erosion, transport materials along with stream flow, and deposit them as solids. Normally it is desirable for streams used as a source of water to have minimal sedimentary material, which has to be removed during water treatment.
Most rivers, once free flowing and unimpeded by human intervention, have been modified by humans to generate power, for water supply, and reduce effects of flooding. Beginning with impoundment of water from the Owens Valley in northern California and extending to the Feather, Sacramento, and Colorado rivers, Los Angeles’ voracious thirst for water is served by a vast system of dams, canals, and tunnels. Diversion of water from the Owens valley has turned a formerly productive agricultural area into one of limited agricultural use. Many of the adverse effects of water diversion have resulted from dams built to confine rivers. As a result of dam construction, many once beautiful river valleys have been covered with water and productive farmland in river valleys has been lost. For example, the beautiful Hetch-Hetchy Valley in Yosemite National Park in California has been inundated since the early 1900s by construction of a dam built to provide water and hydroelectric power to San Francisco. Serious proposals are now being considered to remove the dam and restore the valley to its former beauty.
Much of the water that humans use comes from standing bodies of water including natural lakes and reservoirs constructed by placing dams on rivers. Wetlands are bodies of water shallow enough to support the growth of bottom-rooted plants. Estuaries form where fresh river water flows into oceans. They have unique physical, chemical, and biological properties because of the mixing of fresh water and saltwater. Wetlands and estuaries are important breeding grounds for a number of organisms and it is crucial that they be preserved. Many wetland areas have been drained for agricultural land and an important effort in conservation is their preservation and restoration.
Artesian Wells
Artesian aquifers are those confined by dense clay or shale such that water flows naturally from an artesian well drilled into them. The name comes from the Roman city of Artesum at the site of the French town of Artois known for the free-flowing water from wells dug in the Middle Ages. Artesian wells are often highly prized sources of water. Some modern day bottling companies have leased or purchased sites of artesian wells and advertise their product as artesian water, although it does not differ in quality from pumped well water. Many artesian wells have lost their free-flowing qualities because of depletion of their water resource.
Water Availability
Fresh surface water and groundwater are the major sources for human use. Desalinated seawater is a growing source of fresh water in some areas that do not have access to this valuable resource. Most seawater desalination plants are in the Middle East, an arid region with generally abundant sources of energy that can be used for desalination. The world’s largest desalination plant is the Jebel Ali multi-stage flash distillation unit in the United Arab Emirates that can produce 300 million cubic meters of potable water per year. There are significant resources of brackish (saline) groundwater throughout the world. In many cases this water has a lower salt content than seawater making it easier and more economical to desalinate.
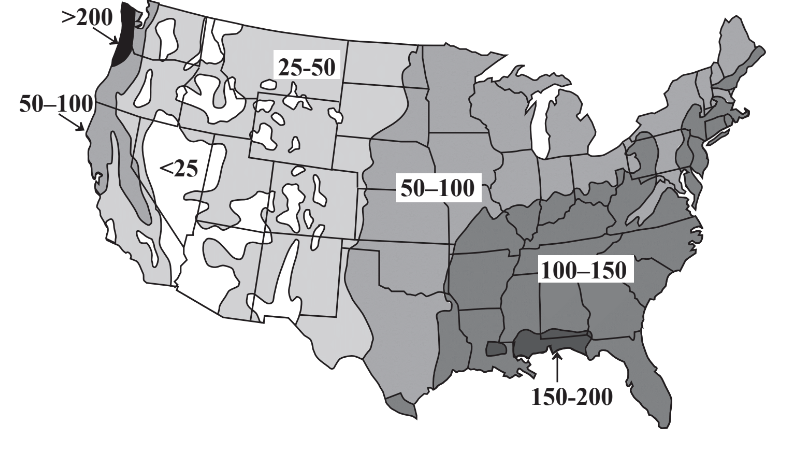
A major concern regarding water’s availability is its uneven distribution with respect to time and location. Some areas experience tremendous rainfall and flooding during wet (monsoon) seasons with dry conditions in between. Whereas these fluctuations are predictable and compensation may be made for them, greater problems occur with long-term droughts. Regions of Africa are periodically afflicted with droughts that last for several years, killing crops and animals and inflicting great hardship on the people in the region. Evidence from tree rings indicates a pre-Columbian drought of almost three centuries duration in what is now the southwestern US. Uneven geographic distribution of water occurs throughout the world and is illustrated for the continental US in Figure \(\PageIndex{3}\). It is seen that precipitation is generally adequate in the eastern part of the country, although damaging droughts do occur in this region. However, the western continental US has a shortage of precipitation with essentially permanent drought conditions in some regions including Nevada, Arizona, and southern California. The problem is exacerbated by the popularity of these regions as areas in which people want to live and the demands that they put on limited water resources.
Common Ions Present in Natural Water
As stated above, the fact that water molecules are highly polar allows water to dissolve a variety of ionic compounds. Minerals found in rocks and soil usually dissolve in natural water bodies such as lakes, rivers, springs, and underground waterways (groundwaters). Calcium carbonate, CaCO3, is one of the most common inorganic compounds in the Earth's crust. It is the ingredient for both calcite and aragonite. These two minerals have different crystal structures and appearances (Figure \(\PageIndex{4}\)).


Dust particles and ions present in the air are nucleation centers for water drops. Thus, waters from rain and snow also contain cations such as Ca2+, Mg2+, Na+, K+, and NH4+. These cations are balanced by anions like HCO3-, SO4-, NO2-, Cl-, and NO3-. The pH of rain is between 5.5 and 5.6 because of the presence of some of these ions. Rain and snow waters eventually become river or lake waters. When the rain or snow waters fall, they interact with vegetation, top soil, bed rock, river bed and lake bed, dissolving whatever is soluble. Bacteria, algae, and water insects also thrive. The most common ions in lake and river waters are the same as those present in rainwater, but at higher concentrations. The pH of these waters depends on the river bed and lake bed. All of these particles and ions can eventually wind up in the oceans where the ion concentration is generally greater than in fresh water sources. Table \(\PageIndex{1}\) lists the major ions present in seawater. The composition does vary depending on region, depth, latitude, and water temperature. Waters at the river mouths contain less salt. If the ions are utilized by living organism, its contents vary according to the populations of organisms.
| Species | Cl− | Na+ | SO42- | Mg2+ | Ca2+ | K+ | HCO3− | Br− | Sr2+ | BO43− | F− | H4SiO4 | H+ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/kg | 10,760 | 2,710 | 2,710 | 1,290 | 411 | 399 | 142 | 67 | 8 | 4.5 | 1.3 | 0.5-10 | 10−8.35 |
Hard Water
Waters containing Ca2+ and Mg2+ ions are usually called hard water. Hard water is high in dissolved minerals and, while other metal cations may be present in the water as well, measuring the concentration of Ca2+ and Mg2+ in the water will give a useful indication of how much dissolved mineral is present. General guidelines for classification of waters are: 0 to 60 mg/L as calcium carbonate is classified as soft; 61 to 120 mg/L as moderately hard; 121 to 180 mg/L as hard; and more than 180 mg/L as very hard. Water hardness varies throughout the United States (Figures \(\PageIndex{5}\) and \(\PageIndex{6}\)).


For those with hard water, the metal cations present can have unpleasant consequences. These cations, especially Ca2+, tend to react with soap to form soap scum. This can be felt as a residue when washing hands and spots or film on glasses after going through a dishwasher. It also means that more soap is usually needed in order to do the same amount of cleaning. In addition, when hard water is heated, solid calcium carbonate can form as scale in water heaters and boilers or as deposits in pipes. Both of these effects lower the efficiency and life span of the equipment.
Summary
- Water's polarity and hydrogen bonding capability allow it to absorb large quantities of heat while being heated and when undergoing phase changes.
- Groundwater is often contained in aquifers between layers of rock. This water is able to flow based on the porosity of the ground and its permeability and can be tapped using wells to provide the water for consumption.
- Surface water found in streams and lakes is also available for use.
- Changing precipitation is causing some areas to have excess water and other areas to experience drought. Communities need to find ways to adapt to water needs to ensure that there is adequate potable water for everyone.
- As water flows through soil, it can dissolve various minerals resulting in a number of different metal cations being dissolved in the water. The most prevalent of these ions are Ca2+ and Mg2+. The amount of these ions in the water is referred to as the hardness of the water.
Contributors and Attributions
-
Chung (Peter) Chieh (Professor Emeritus, Chemistry @ University of Waterloo)
-
Stanley E. Manahan (University of Missouri)
-
Vicki MacMurdo (Anoka-Ramsey Community College)