16.2: Chemistry and the Environment
- Page ID
- 434968
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Identify the five spheres of the environment and how they can interact with each other.
- Identify the areas of environmental chemistry that relate to each of the five spheres.
Environmental Spheres
The environment consists of our surroundings, which may affect us and which, in turn, we may affect. Obviously the chemical nature and processes of matter in the environment are important. Compared to the generally well-defined processes that chemists study in the laboratory, those that occur in the environment are rather complex and must be viewed in terms of simplified models. A large part of this complexity is due to the fact that the environment consists of the five overlapping and interacting spheres — the atmosphere, the hydrosphere, the geosphere, the biosphere, and the anthrosphere — shown from the viewpoint of their chemical interactions in Figure \(\PageIndex{1}\). In order to better understand the chemistry that occurs in these spheres, they are briefly described here.
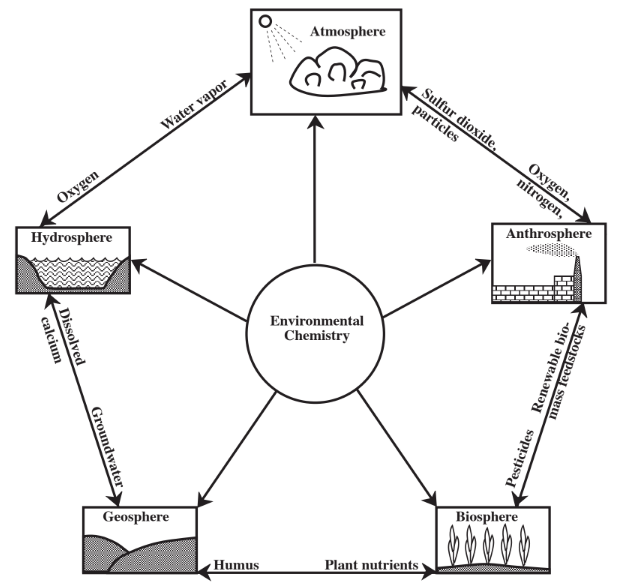
The atmosphere is a very thin layer compared to the size of Earth, with most atmospheric gases lying within a few kilometers of sea level. In addition to providing oxygen for living organisms, the atmosphere provides carbon dioxide required for plant photosynthesis, and nitrogen that organisms use to make proteins. The atmosphere serves a vital protective function in that it absorbs highly energetic ultraviolet radiation from the sun that would kill living organisms exposed to it. A particularly important part of the atmosphere in this respect is the stratospheric layer of ozone, an ultraviolet-absorbing form of elemental oxygen. In addition, the atmosphere stabilizes Earth’s surface temperature by absorbing infrared radiation (heat) which the Earth would otherwise lose by radiating it out to space. The atmosphere also serves as the medium by which solar energy, that falls with greatest intensity in equatorial regions, is redistributed away from the Equator. It is the medium in which water vapor, evaporated from oceans as the first step in the hydrologic cycle, is transported over land masses to fall as rain over land.
Earth’s water is contained in the hydrosphere. Although frequent reports of torrential rainstorms and flooded rivers produced by massive storms might give the impression that a large fraction of Earth’s water is fresh water, in reality more than 97% of the water on Earth is seawater in the oceans. Of the remaining 3% that is fresh water, most is present as ice in polar ice caps and glaciers. A small fraction of the total water is present as vapor in the atmosphere. The remaining liquid fresh water is what is available for growing plants and other organisms and for industrial uses. This water may be present on the surface as lakes, reservoirs, and streams, or it may be underground as groundwater.
The solid part of earth, the geosphere, includes all rocks and minerals. A particularly important part of the geosphere is soil which supports plant growth, the basis of food for all living organisms. The lithosphere is a relatively thin solid layer extending from Earth’s surface to depths of 50–100 km. The even thinner outer skin of the lithosphere known as the crust is composed of relatively lighter silicate-based minerals. It is the part of the geosphere that is available to interact with the other environmental spheres and that is accessible to humans.
The biosphere is composed of all living organisms. For the most part, these organisms live on the surface of the geosphere on soil, or just below the soil surface. The oceans and other bodies of water support high populations of organisms. Some life forms exist at considerable depths on ocean floors. In general, though, the biosphere is a very thin layer at the interface of the geosphere with the atmosphere. The biosphere is involved with the geosphere, hydrosphere, and atmosphere in biogeochemical cycles through which materials such as nitrogen and carbon are circulated.
Through human activities, the anthrosphere, that part of the environment made and operated by humans, has developed strong interactions with the other environmental spheres. Many examples of these interactions could be cited. By cultivating large areas of soil for domestic crops, humans modify the geosphere and influence the kinds of organisms in the biosphere. Humans divert water from its natural flow, use it, sometimes contaminate it, then return it to the hydrosphere. Emissions of particles to the atmosphere by human activities affect visibility and other characteristics of the atmosphere. The emission of large quantities of carbon dioxide to the atmosphere by combustion of fossil fuels are modifying the heat-absorbing characteristics of the atmosphere to the extent that global warming is almost certainly taking place. The anthrosphere perturbs various biogeochemical cycles.
Environmental Chemistry and the Five Spheres
Environmental chemistry, an important branch of chemical science, studies the sources, reactions, transport, effects, and fates of chemical species in water, soil, air, and living environments and the effects of technology on them. Figure \(\PageIndex{1}\), which shows the five environmental spheres, may provide an idea of the complexity of environmental chemistry as a discipline. Enormous quantities of materials and energy are continually exchanged among the five environmental spheres. In addition to variable flows of materials, there are variations in temperature, intensity of solar radiation, mixing, and other factors, all of which strongly influence chemical conditions and behavior.
There are several highly interconnected and overlapping categories of environmental chemistry. Atmospheric chemistry is the branch of environmental chemistry that considers chemical phenomena in the atmosphere. Two things that make this chemistry unique are the extreme dilution of important atmospheric chemicals and the influence of photochemistry. Photochemistry occurs when molecules absorb photons of high-energy visible light or ultraviolet radiation, become energized (“excited”), and undergo reactions that lead to a variety of products, such as photochemical smog. In addition to reactions that occur in the gas phase, many important atmospheric chemical phenomena take place on the surfaces of very small solid particles suspended in the atmosphere and in droplets of liquid in the atmosphere. Although no significant atmospheric chemical reactions are mediated by organisms in the atmosphere, microorganisms play a strong role in determining species that get into the atmosphere. As examples, bacteria growing in the absence of oxygen, such as in cows’ stomachs and under water in rice paddies, are the single greatest source of hydrocarbon in the atmosphere because of the large amounts of methane that they emit. The greatest source of organic sulfur compounds in the atmosphere consists of microorganisms in the oceans that emit dimethyl sulfide.
Aquatic chemistry deals with chemical phenomena and processes in water. Aquatic chemical processes are very strongly influenced by microorganisms in the water, so there is a strong connection between the hydrosphere and biosphere insofar as such processes are concerned. Aquatic chemical processes occur largely in “natural waters” consisting of water in oceans, bodies of fresh water, streams, and underground aquifers. These are places in which the hydrosphere can interact with the geosphere, biosphere, and atmosphere and is often subjected to anthrospheric influences.
Chemical processes that occur in the geosphere involving minerals and their interactions with water, air, and living organisms are addressed by the topic of geochemistry. A special branch of geochemistry, soil chemistry, deals with the chemical and biochemical processes that occur in soil.
Environmental biochemistry addresses biologically mediated processes that occur in the environment. Such processes include the biodegradation of organic waste materials in soil or water and processes within biogeochemical cycles, such as denitrification, which returns chemically bound nitrogen to the atmosphere as nitrogen gas. The toxic effects of chemicals on a variety of living organisms are of utmost concern to chemists and the public as well.
Although there is not a formally recognized area of chemistry known as “anthrospheric chemistry,” most of chemical science and engineering developed to date deals with chemistry carried out in the anthrosphere. Included is industrial chemistry, which is very closely tied to the practice of green chemistry. A good way to view “anthrospheric chemistry” from a green chemistry perspective is within the context of industrial ecology. Industrial ecology considers industrial systems in a manner analogous to natural ecosystems. In a system of industrial ecology, various manufacturing and processing operations carry out “industrial metabolism” on materials. A successful industrial ecosystem is well balanced and diverse, with various enterprises that generate products for each other and use each other’s products and potential wastes. A well-functioning industrial ecosystem recycles materials to the maximum extent possible and produces little — ideally no — wastes. Therefore, a good industrial ecosystem is a green chemical system.
Summary
- The environment consists of five overlapping and interacting spheres: the atmosphere, the hydrosphere, the geosphere, the biosphere, and the anthrosphere.
- Environmental chemistry studies the sources, reactions, transport, effects, and fates of chemical species in water, soil, air, and living environments.
- There are categories of environmental chemistry which deal with each of the five spheres of the environment.
Contributions & Attributions
This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
- Stanley E. Manahan (University of Missouri)
- Vicki MacMurdo (Anoka-Ramsey Community College)

