16.11: Groundwater Contamination
- Page ID
- 434977
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Define groundwater contamination.
- Describe the impact of human activities on water quality.
- List the various groundwater contaminants and their sources.
Water is a resource that is required for all life, including human life. In 2010, the UN General Assembly explicitly recognized the human right to water and sanitation. For water to be useful for drinking and irrigation, it must not be polluted beyond certain thresholds. According to the World Health Organization, 2 billion people lived in water-stressed communities without safely managed drinking-water services in 2020. At the same time, over 1.7 billion people still did not have basic sanitation services, which is defined as having access to a public sewage system, septic tank, or even a simple pit latrine. Each year approximately 1.7 million people die from diarrheal diseases associated with unsafe drinking water, inadequate sanitation, and poor hygiene. Almost all of these deaths are in developing countries, and around 90% of them occur among children under the age of 5. Compounding the water crisis is the issue of social justice; poor people more commonly lack clean water and sanitation than wealthy people in similar areas. Globally, improving water safety, sanitation, and hygiene could prevent up to 9% of all disease and 6% of all deaths.
In addition to the global waterborne disease crisis, chemical pollution from agriculture, industry, cities, and mining threatens global water quality. In Gallup public polls conducted over the past decade, Americans consistently put water pollution and water supply as the top environmental concerns over issues such as air pollution, deforestation, species extinction, and global warming.
Groundwater contamination (also called groundwater pollution) occurs when pollutants are released to the ground and make their way down into groundwater. This type of water pollution can also occur naturally due to the presence of a minor and unwanted constituent, contaminant, or impurity in the groundwater, in which case it is more likely referred to as contamination rather than pollution.
How Does Groundwater Become Contaminated?
Water pollution is the contamination of water by an excess amount of a substance that can cause harm to human beings and/or the ecosystem. The level of water pollution depends on the abundance of the pollutant, the ecological impact of the pollutant, and the use of the water. Pollutants are derived from biological, chemical, or physical processes. Although natural processes such as volcanic eruptions or evaporation sometimes can cause water pollution, most pollution is derived from human, land-based activities. Water pollutants can move through different water reservoirs as the water carrying them progresses through stages of the water cycle.
Pollutants enter water supplies from point sources, which are readily identifiable and relatively small locations, or nonpoint sources, which are large and more diffuse areas (Figure \(\PageIndex{1}\)). Point sources of pollution include animal factory farms that raise a large number and high density of livestock such as cows, pigs, and chickens. Also included are pipes from factories or sewage treatment plants. Combined sewer systems that have a single set of underground pipes to collect both sewage and storm water runoff from streets for wastewater treatment can be major point sources of pollutants. During heavy rain, storm water runoff may exceed sewer capacity, causing it to back up and spilling untreated sewage directly into surface waters.


If the pollutant enters an aquifer, it often creates a contaminant plume within the aquifer. Movement of water and dispersion within the aquifer spreads the pollutant over a wider area. Its advancing boundary, often called a plume edge, can intersect with groundwater wells or move into surface water through seeps and springs, making the water supplies unsafe for humans and wildlife. The movement of the plume, called a plume front, may be analyzed through a hydrological transport model or groundwater model. Analysis of groundwater pollution may focus on soil characteristics and site geology, hydrogeology, hydrology, and the nature of the contaminants.
Types of Contamination
Several kinds of pollutants are detrimental to water that is a source of water supply. A variety of impurities can affect taste, odor, and color. Sediments require removal and sometimes special measures are needed to coagulate and settle colloidal suspended matter. Acidity, alkalinity, and salinity at excessive levels are water pollutants.
Several trace metals occur naturally in certain rock formations and can enter in the environment from natural processes such as weathering. However, industrial activities such as mining, metallurgy, solid waste disposal, paint and enamel works, etc. can lead to elevated concentrations of toxic metals including lead, cadmium, and chromium. These contaminants have the potential to make their way into groundwater. The migration of metals (and metalloids) in groundwater will be affected by several factors, in particular by chemical reactions which determine the partitioning of contaminants among different phases and species. Thus, the mobility of metals primarily depends on both the pH of the groundwater and the presence of ions in it with which they can react.
In some cases radionuclides occur as water pollutants. Leakage of radioactive tritium, a form of hydrogen, has gotten into water from reactors. The radionuclide of most concern usually is radium from natural sources and a number of groundwater sources of water have been discontinued because of the presence of radium.
Arsenic and fluoride
Arsenic and fluoride have been recognized by the World Health Organization (WHO) as the most serious inorganic contaminants in drinking-water on a worldwide basis. Inorganic arsenic is the most common type of arsenic in soil and water. The metalloid arsenic can occur naturally in groundwater, as seen most frequently in Asia, including in China, India and Bangladesh. In the Ganges Plain of northern India and Bangladesh, severe contamination of groundwater by naturally occurring arsenic affects 25% of water wells in the shallower of two regional aquifers. Groundwater in these areas is also contaminated by the use of arsenic-based pesticides. Arsenic in groundwater can also be present where there are mining operations or mine waste dumps that will leach arsenic.
Natural fluoride in groundwater is of growing concern as deeper groundwater is being used, "with more than 200 million people at risk of drinking water with elevated concentrations." Fluoride can especially be released from acidic volcanic rocks and dispersed volcanic ash when water hardness is low. High levels of fluoride in groundwater is a serious problem in the Argentinean Pampas, Chile, Mexico, India, Pakistan, the East African Rift, and some volcanic islands (Tenerife). In areas that have naturally occurring high levels of fluoride in groundwater which is used for drinking water, both dental and skeletal fluorosis can be prevalent and severe.
Nitrate
Nitrate is the most common chemical contaminant in the world's groundwater and aquifers. In some low-income countries, nitrate levels in groundwater are extremely high, causing significant health problems. It is also stable (it does not degrade) under high oxygen conditions. Elevated nitrate levels in groundwater can be caused by on-site sanitation, sewage sludge disposal and agricultural activities. It can therefore have an urban or agricultural origin.
Nitrate levels above 10 mg/L (10 ppm) in groundwater can cause "blue baby syndrome" (acquired methemoglobinemia). Drinking water quality standards in the European Union stipulate less than 50 mg/L for nitrate in drinking water. However, the linkages between nitrates in drinking water and blue baby syndrome have been disputed in other studies. The syndrome outbreaks might be due to other factors than elevated nitrate concentrations in drinking water.
Biochemical Oxygen Demand
Some pollutants are picked up by water as a natural consequence of its use in municipal water systems, which take in potable water fit to drink and discharge wastewater. One of the main components of this wastewater consists of sewage including human wastes, food wastes, and other substances that get into water through bathrooms and kitchens. A major component of these water pollutants consists of biodegradable organic matter, abbreviated here as {CH2O} and commonly called biochemical oxygen demand, BOD, which rapidly depletes receiving water of its dissolved oxygen by the following microbially-mediated process:
\[\left\{{\mathrm{CH}}_2\mathrm O\right\}\;+\:{\mathrm O}_2\;\rightarrow\;{\mathrm{CO}}_2\;+\:{\mathrm H}_2\mathrm O\]
Oxygen-demanding waste is an extremely important pollutant to ecosystems. Most surface water in contact with the atmosphere has a small amount of dissolved oxygen which is needed by aquatic organisms for cellular respiration. Too much decaying organic matter in water is a pollutant because it removes oxygen from water, which can kill fish, shellfish, and aquatic insects. An unpolluted water body with respect to BOD is a turbulent river that flows through a natural forest. Turbulence continually brings water in contact with the atmosphere where the O2 content is restored. The dissolved oxygen content in such a river ranges from 10 to 14 ppm O2, BOD is low, and clean-water fish such as trout thrive. A polluted water body with respect to oxygen is a stagnant deep lake in an urban setting with a combined sewer system. This system favors a high input of dead organic carbon from sewage overflows and limited chance for water circulation and contact with the atmosphere. In such a lake, the dissolved O2 content is ≤5 ppm O2, BOD is high, and low O2-tolerant fish, such as carp and catfish, dominate.
Excessive plant nutrients, particularly nitrogen (NH4+, NO3-) and phosphorous (as phosphates H2PO4-, HPO42-), are pollutants closely related to oxygen-demanding waste. Aquatic plants require about 15 nutrients for growth, most of which are plentiful in water. Nitrogen and phosphorus are called limiting nutrients, however, because they usually are present in water at low concentrations and therefore restrict the total amount of plant growth. This explains why nitrogen and phosphorus are major ingredients in most fertilizer. High concentrations of these elements from human sources (mostly agricultural and urban runoff including fertilizer, sewage, and phosphorus-based detergent) can cause cultural eutrophication, which leads to the rapid growth of aquatic producers, particularly algae. Thick mats of floating algae or rooted plants lead to a form of water pollution that damages the ecosystem by clogging fish gills and blocking sunlight. A small percentage of algal species produce toxins that can kill animals, including humans. Exponential growths of these algae are called harmful algal blooms. When the prolific algal layer dies, it becomes oxygen-demanding waste, which can create very low O2 concentrations in the water (< 2 ppm O2), a condition called hypoxia. This results in a dead zone because it causes death from asphyxiation to organisms that are unable to leave that environment. An estimated 50% of lakes in North America, Europe, and Asia are negatively impacted by cultural eutrophication. In addition, the size and number of marine hypoxic zones have grown dramatically over the past 50 years including a very large dead zone located offshore Louisiana in the Gulf of Mexico. Cultural eutrophication and hypoxia are difficult to combat, because they are caused primarily by nonpoint source pollution, which is difficult to regulate, and nitrogen and phosphorus, which are difficult to remove from wastewater.
Organic compounds
A variety of organic compounds occur as water pollutants. These include industrial chemicals, petroleum products (especially significant in light of the notorious 2010 leak from the Deepwater Horizon oil spill that dumped as much as 5 million gallons of crude oil into the Gulf of Mexico and caused billions of dollars in pollution damage to the Gulf and its shore areas), polychlorinated biphenyls (PCBs, now banned from manufacture but still sometimes encountered, especially in sediments), and dioxin. Polyfluoroalkyls (PFAs) and volatile organic compounds (VOCs) are a dangerous contaminant of groundwater that come from industry. They are generally introduced to the environment through careless industrial practices. Many of these compounds were not known to be harmful until the late 1960s and it was some time before regular testing of groundwater identified these substances in drinking water sources.
Organic pollutants can also be found in groundwater as insecticides and herbicides. As with many other synthetic organic compounds, most pesticides have very complex molecular structures. This complexity determines the water solubility, adsorption capacity, and mobility of pesticides in the groundwater system. Thus, some types of pesticides are more mobile than others so they can more easily reach a drinking-water source.
Pharmaceuticals
Since the 1990s, water contamination by pharmaceuticals has been an environmental issue of concern. The use of pharmaceuticals and personal care products (PPCPs) is on the rise with an estimated increase from 2 billion to 3.9 billion annual prescriptions between 1999 and  2009 in the United States alone. Popular pharmaceuticals such as antibiotics, anti-inflammatories, antidepressants, decongestants, tranquilizers, etc. are normally found in treated wastewater (Figure \(\PageIndex{2}\)). This wastewater is discharged from the treatment facility and often makes its way into the aquifer or other source of surface water used for drinking water. Trace amounts of pharmaceuticals in both groundwater and surface water are far below what is considered dangerous or of concern in most areas, but it could be an increasing problem as population grows and more reclaimed wastewater is utilized for municipal water supplies.
2009 in the United States alone. Popular pharmaceuticals such as antibiotics, anti-inflammatories, antidepressants, decongestants, tranquilizers, etc. are normally found in treated wastewater (Figure \(\PageIndex{2}\)). This wastewater is discharged from the treatment facility and often makes its way into the aquifer or other source of surface water used for drinking water. Trace amounts of pharmaceuticals in both groundwater and surface water are far below what is considered dangerous or of concern in most areas, but it could be an increasing problem as population grows and more reclaimed wastewater is utilized for municipal water supplies.

The major route for pharmaceutical residues to reach the aquatic environment is most probably by excretion from patients undergoing pharma treatment. Since many pharmaceutical substances are not metabolized in the body, they may be excreted in biologically active form, usually via the urine. Furthermore, many pharmaceutical substances are not fully taken up from the intestine (following oral administration in patients) into their blood stream. The fraction not taken up into the blood stream will remain in the gut and eventually be excreted via the feces. Hence, both urine and feces from treated patients contain pharmaceutical residues. Between 30 and 90% of the orally administered dose is generally excreted as active substance in the urine. Figure \(\PageIndex{3}\) illustrates how PPCPs then enter into the aquatic environment through this type of individual human activity.
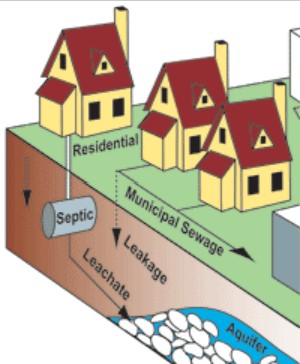
An additional source to environmental pollution with pharmaceuticals is improper disposal of unused or expired drug residues (pharma residues), either by individual consumers or larger facilities. In 2009, an investigative report by Associated Press concluded that U.S. manufacturers had legally released 271 million pounds of compounds used as drugs into the environment, 92% of which was the industrial chemicals phenol and hydrogen peroxide, which are also used as antiseptics. It could not distinguish between drugs released by manufacturers as opposed to the pharmaceutical industry. It also found that an estimated 250 million pounds of pharmaceuticals and contaminated packaging were discarded by hospitals and long-term care facilities. In Europe, the input of pharmaceutical residues via domestic (individual) waste water is estimated to be around 80%, whereas 20% is coming from hospitals.
Pharmaceuticals used in veterinary medicine, or as additives to animal food, pose a different problem since they are excreted into soil or possibly open surface waters. It is well known that such excretions may affect terrestrial organisms directly, leading to extinction of exposed species (e.g. dung-beetles). Lipid-soluble pharma residues from veterinary use may bind strongly to soil particles, with little tendency to leak out to ground water or to local surface waters. More water-soluble residues may be washed out with rain or melting snow and reach both ground water and surface water streams.
Once in the water, pharma residues can have diverse, subtle effects on organisms, although research is still limited. Because of the high solubility of most PPCPs, aquatic organisms are especially vulnerable to their effects. The increased presence of estrogen and other synthetic hormones in waste water due to birth control and hormonal therapies has been linked to increased feminization of exposed fish and other aquatic organisms. The chemicals within these PPCP products could either affect the feminization or masculinization of different fishes, therefore affecting their reproductive rates. While the full effects of most PPCPs on the environment are not understood, there is concern about the potential they have for harm because they may act unpredictably when mixed with other chemicals from the environment or concentrate in the food chain. Additionally, some PPCPs are active at very low concentrations and are often released continuously in large or widespread quantities.
Water Borne Diseases
Waterborne diseases are conditions caused by pathogenic micro-organisms that are transmitted in water. These diseases can be spread while bathing, washing, drinking water, or by eating food exposed to contaminated water. While diarrhea and vomiting are the most commonly reported symptoms of waterborne illness, other symptoms can include skin, ear, respiratory, or eye problems. Waterborne diseases are impacted by a country's economy and also impact the economy by being costly to deal with.
The lack of proper sanitation measures, as well as improperly placed wells, can lead to drinking water contaminated with pathogens carried in feces and urine. Such fecal-oral transmitted diseases include typhoid, cholera and diarrhea. Microorganisms causing diseases that characteristically are waterborne prominently include protozoa and bacteria, many of which are intestinal parasites, or invade the tissues or circulatory system through walls of the digestive tract. Various other waterborne diseases are caused by viruses.
Deep, confined aquifers are usually considered the safest source of drinking water with respect to pathogens. Pathogens from treated or untreated wastewater can contaminate certain types of aquifers, especially shallow ones.
The table below shows water-borne diseases that can result from viruses, bacteria, and parasites. In some cases, vaccines are available. When eating, drinking, or swimming, it is important to be aware of how you could be affected by these pathogens. In addition, proper handling of foods and beverages could reduce your risk of developing one or more of the following health problems.
| Pathogen Name | Pathogen Type | Source | Health problem | Prevention/Treatment |
|---|---|---|---|---|
| Giardia | Parasite | Fecal contamination and uncooked food | Vomiting, diarrhea, and cramps | Medication afterward |
| Cryptosporidium | Parasite | Fecal contamination | Vomiting, diarrhea, fever, and cramps | Medication afterward |
| Typhoid | Bacteria | Fecal contamination | High fever, stomach pains, headache, and rash | Vaccination/Antibiotics |
| E. coli | Bacteria | Fecal contamination | Diarrhea and cramps | Fluids |
| Legionella | Bacteria | Found naturally in heated water | Causes Legionnaires (a type of pneumonia) | Medications afterward |
| Cholera | Bacteria | Related to fecal contamination or undercooked or raw shellfish | Diarrhea | Vaccine/Rehydration, antibiotics, and Zinc |
| Hepatitis A | Virus | Contaminated food and water | Vomiting, dark urine, and yellowing of the eyes. | Vaccination/Fluids |
| Polio | Virus | Fecal contamination | Flu symptoms, paralysis | Vaccination |
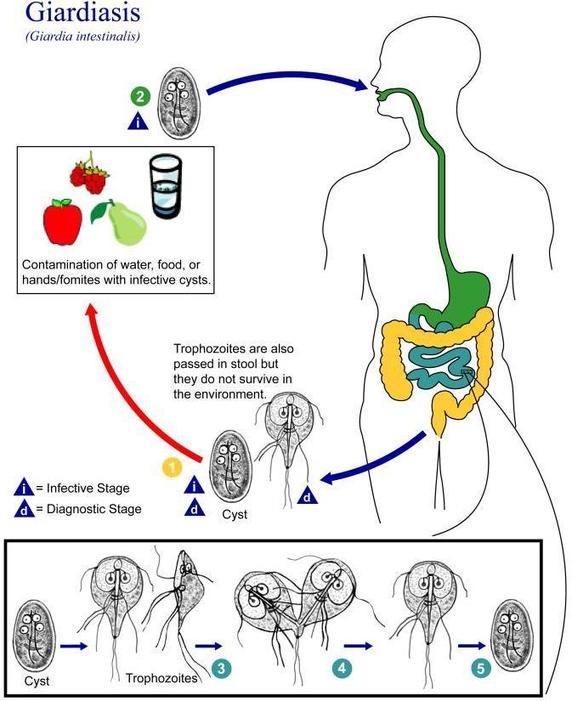
Figure \(\PageIndex{4}\) shows how a person might contract Giardiasis from giardia, a parasite. This particular pathogen can live in a body up to six months. Once detected through a stool sample, a patient can be prescribed specific antibiotics like Flagyl to treat the infection. Unfortunately, there is no vaccine for preventing Giardiasis.
Summary
- Water pollution is the contamination of water by an excess amount of a substance that can cause harm to human beings and/or the ecosystem.
- Pollutants enter water supplies from point sources, which are readily identifiable and relatively small locations, or nonpoint sources, which are large and more diffuse areas.
- Contaminants found in groundwater cover a broad range of physical, inorganic chemical, organic chemical, bacteriological, and radioactive parameters.
- Organic matter, as well as phosphates and nitrates from human and farm animal waste, support the growth of algae and microorganisms, including bacteria.
- Eutrophication is an increase in the concentration of chemical nutrients in an ecosystem to an extent that increases the primary productivity of the ecosystem. Depending on the degree of eutrophication, subsequent negative environmental effects such as anoxia (oxygen depletion) and severe reductions in water quality may occur, affecting fish and other animal populations.
- A contaminant of growing concern is pharmaceuticals which enter waste water streams either through improper disposal of expired prescriptions or through excrement.
- A variety of diseases can be found in water due to a lack of proper sanitation measures or improper placement of wells.
Contributors and Attributions
- Essentials of Environmental Science by Kamala Doršner is licensed under CC BY 4.0. Modified from the original by Matthew R. Fisher.
- Vicki MacMurdo (Anoka-Ramsey Community College)
- Wikipedia
- US EPA
- US Geological Survey (USGS)

