16.12: Water Purification
- Page ID
- 434978
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Describe ways in which hard water may be softened.
- Identify steps that can be taken to minimize the release of contaminants into groundwater.
- Describe ways in which contaminants can be removed from municipal water sources.
- Suggest ways to provide potable water for those in areas currently without clean water.
We saw in Section 16.10 that natural water often contains a variety of metal ions that it picks up from dissolving minerals as it makes its way through the ground. These metal ions can affect the taste and color of the water. They can also cause build up in pipes and lessen the effectiveness of soaps. In addition, Section 16.11 described a variety of pollutants which can be present in the water, many of which can have adverse health effects. In order for water to be safe for drinking, it often needs to undergo some sort of purification process. The type of process used depends on the compounds which need to be removed from the water and the amount of water being purified.
Softening Water
In an effort to mitigate the effects of hard water, many choose to treat the water in order to "soften" it. Softening water simply means removing the cations such as Ca2+ and Mg2+ which tend to precipitate out and replace them with more soluble ions like Na+. There are a number of ways to accomplish this depending on the scale required and the amount one is willing to spend.
For larger scale municipal water systems, lime-soda softening can be used to remove Ca2+ and Mg2+ by adding a calculated amount of hydrated lime, Ca(OH)2, and sodium carbonate, Na2CO3. The lime will react with Ca2+ ions:
\[\mathrm{Ca}^{2+}\:+\:2\;\mathrm{HCO}_3^-\;+\:\mathrm{Ca}{(\mathrm{OH})}_{2(s)}\;\rightarrow\;2\;{\mathrm{CaCO}}_{3(s)}\;+\:2\;{\mathrm H}_2\mathrm O\]
Adding more lime causes the pH of water to increase, and as a result, magnesium ions are removed by the reaction:
\[\mathrm{Mg}^{2+}\;+\:\mathrm{Ca}{(\mathrm{OH})}_{2(s)}\;\rightarrow\;\mathrm{Mg}{(\mathrm{OH})}_{2(s)}\;+\:\mathrm{Ca}^{2+}\]
The extra calcium ions can be removed by the addition of the sodium carbonate, also known as soda ash.
\[{\mathrm{Na}}_2{\mathrm{CO}}_3\;\rightarrow\;2\;\mathrm{Na}^+\:+\:{\mathrm{CO}}_3^{2-}\]
\[\mathrm{Ca}^{2+}\:+\:{\mathrm{CO}}_3^{2-}\;\rightarrow\;{\mathrm{CaCO}}_{3(s)}\]
Due to the number of solid products formed, this type of treatment will often have a series of ponds for settling out solids and filters for removing them. These solids can then be used for other purposes such as the formation of cement. The water tends to be quite basic after the process is completed and thus the pH must be lowered after treatment before continuing on to homes and businesses.
Many households will choose to have water softeners to soften the municipal water or well water that comes into their homes. Today, most water softeners are using zeolites and employing ion exchange techniques to soften hard water. Zeolites are a group of hydrated crystalline aluminosilicates found in certain volcanic rocks. The tetrahedrally coordinated aluminum and silicon atoms form AlO4 and SiO4 tetrahedral groups. They interconnect to each other sharing oxygen atoms, forming cage-type structures as shown in Figure \(\PageIndex{1}\). Whatever kind is used, the crystal structure of zeolites contains large cages. The cages are connected to each other forming a framework with many cavities and channels. Both positive and negative ions can be trapped in these cavities and channels.
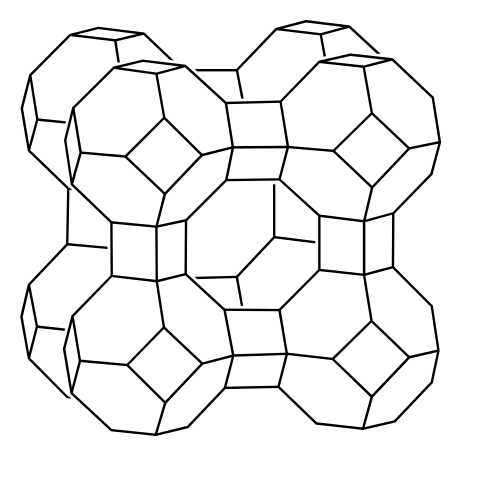


For each oxygen that is not shared in the AlO4 and SiO4 tetrahedral groups, a negative charge is left on the group. These negative charges are balanced by trapping alkali metal and alkaline earth metal ions. When more cations are trapped, hydroxide and chloride ions will remain in the cavities and channels of the zeolites.
To prepare a zeolite for water treatment, they are soaked in concentrated NaCl solution. The cavities trap as many sodium ions as they can accommodate. After the treatment, the zeolite is designated as Na-zeolite. Then the salt solution is drained, and the zeolite is washed with water to eliminate the extra salt. When hard water flows through them, calcium and magnesium ions will be trapped by the Na-zeolite. For every Ca2+ or Mg2+ trapped, two Na+ ions are released. The treated water contains a rather high concentration of Na+ ions, but low concentrations of Mg2+ and Ca2+. Thus, zeolite ion exchange converts hard water into soft water.
Preventing the Release of Contaminants
One way to keep groundwater free from contaminants is to prevent them from reaching the aquifers. When spills have happened, either the companies responsible or the government will work to contain the pollutant. If it has entered into the soil, the dirt may be dug up and removed to prevent water from flowing through it and leaching out the contaminant. The soil is then treated to remove the waste before being returned to the ground. Spills that occur in the water can have absorbant materials applied to prevent them from spreading through the water system.
Contaminants that enter through wastewater streams can be minimized by decreasing the amounts that are discharged. Proper destruction of pharma residues (unused or expired medications) should yield products without any pharmaceutical or ecotoxic activity. To facilitate this, European countries have take-back systems in place for such residues (although they are not always utilized to full extent) while in the US only voluntary initiatives on a local basis exist. Incineration at a high temperature (>1000 degrees Celsius) is considered to destroy all pharmacological activity, but even following such incineration residual ashes from the incineration should be properly taken care of. Though most of the waste goes to incineration and people are asked to throw unused or expired pharmaceuticals into the household waste, investigations in Germany showed that up to 24% of liquid pharmaceuticals and 7% of tablets or ointments are disposed of via the toilet or sink and may thus wind up in wastewater treatment.
Treatment of Contaminated Groundwater
With the possibility of so many different contaminates in groundwater, it is imperative that it is treated to make it safe for consumption. For many municipal systems, a key step is filtration or reverse osmosis to create purified water and to concentrate the impurities in a waste stream. If the waste stream contains heavy metals, it can then be reacted with specific chemicals to cause the metals to precipitate out and then they can be isolated by sedimentation. If large organic contaminants are present and trapped by the filtration, they can then be incinerated, whether they are VOCs or pharmaceuticals. Pathogens can be broken down or killed using chlorination or ozone at any point along the treatment process.
For those who live in areas without water treatment systems, some sort of home filtration system is necessary. If water cannot be filtered, boiling can kill some pathogens. However, it will not remove things like heavy metals. There are a number of organizations who focus on providing families with filtration systems or communities with deep wells so that the soil can filter out some of the contaminants. This is especially useful in areas where sewage and other waste waters are released close to the source of drinking water.
Summary
- Water softening exchanges Ca2+ and Mg2+ ions with Na+ ions. The Na+ ions are more soluble and therefore mitigate the effects of hard water.
- When pollutants are found in the ground, large scale, expensive treatment is often required in order to prevent the pollutants from entering the groundwater.
- Municipal water treatment facilities use filtration and reverse osmosis to remove contaminants from water that is then distributed to the community.
- Safe water is a right of all people. Many organizations are working hard to provide clean wells and filtration systems for those who live in areas with contaminated water supplies.
Contributors and Attributions
-
Chung (Peter) Chieh (Professor Emeritus, Chemistry @ University of Waterloo)
-
Vicki MacMurdo (Anoka-Ramsey Community College)
-
Stanley E. Manahan (University of Missouri)

