16.13: Acid Rain
- Page ID
- 434979
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Describe how acid rain is formed.
- Describe the effects of acid rain on the environment and infrastructure.
Acid rain is a term referring to a mixture of wet and dry deposition (deposited material) from the atmosphere (Figure \(\PageIndex{1}\)) containing higher than normal amounts of nitric and sulfuric acids. The precursors, or chemical forerunners, of acid rain formation result from both natural sources, such as volcanoes and decaying vegetation, and man-made sources, primarily emissions of sulfur dioxide (SO2) and nitrogen oxides (NOx) resulting from fossil fuel combustion. Acid rain occurs when these gases react in the atmosphere with water, oxygen, and other chemicals to form various acidic compounds. The result is a dilute solution of sulfuric acid and nitric acid.
\[2\;{\mathrm{SO}}_2\;+\:{\mathrm O}_2\:\rightarrow\;2\;{\mathrm{SO}}_3\]
\[{\mathrm{SO}}_3\;+\:{\mathrm H}_2\mathrm O\:\rightarrow\;\;{\mathrm H}_2{\mathrm{SO}}_4\]
\[2\;{\mathrm{NO}}_2\;+\:{\mathrm H}_2\mathrm O\:\rightarrow\;{\mathrm{HNO}}_2\;+\:{\mathrm{HNO}}_3\]
When sulfur dioxide and nitrogen oxides are released from power plants and other sources, prevailing winds blow these compounds across state and national borders, sometimes over hundreds of miles. Thus acid rain becomes a problem for everyone and not just those who live close to the sources.

Acid rain is measured using the pH scale. As discussed (or will be discussed) in Section 15.7, the lower a substance’s pH, the more acidic it is. Pure water has a pH of 7.0. However, normal rain is slightly acidic because carbon dioxide (CO2) dissolves into it forming dilute carbonic acid solution, giving the resulting mixture a pH of approximately 5.6 at typical atmospheric concentrations of CO2. Acid rain falling in the US has a pH between 4.2 and 4.4, making it more than ten times more acidic.
Effects of Acid Rain
Acid rain causes acidification of lakes and streams and contributes to the damage of trees at high elevations (for example, red spruce trees above 2,000 feet) and many sensitive forest soils. In addition, acid rain accelerates the decay of building materials and paints, including irreplaceable buildings, statues, and sculptures that are part of our world’s cultural heritage. Prior to falling to the earth, sulfur dioxide (SO2) and nitrogen oxide (NOx) gases and their particulate matter derivatives—sulfates and nitrates—contribute to visibility degradation and harm public health.
The ecological effects of acid rain are most clearly seen in the aquatic, or water, environments, such as streams, lakes, and marshes. Most lakes and streams have a pH between 6 and 8, although some lakes are naturally acidic even without the effects of acid rain. Acid rain primarily affects sensitive bodies of water, which are located in watersheds whose soils have a limited ability to neutralize acidic compounds (called “buffering capacity”). Lakes and streams become acidic when the water itself and its surrounding soil cannot buffer the acid rain enough to neutralize it. In areas where buffering capacity is low, acid rain releases aluminum from soils into lakes and streams; aluminum is highly toxic to many species of aquatic organisms. In addition, different aquatic organisms have different sensitivities to acid. At pH 5, most fish eggs cannot hatch. At lower pH levels, some adult fish die. Even if a species of fish or animal can tolerate moderately acidic water, the animals or plants it eats might not. For example, frogs have a critical pH around 4. However, the mayflies they eat may not survive below pH 5.5.
Acid rain causes slower growth, injury, or death of forests as shown in Figure \(\PageIndex{2}\). Of course, acid rain is not the only cause of such conditions. Other factors contribute to the overall stress of these areas, including air pollutants, insects, disease, drought, or very cold weather. In most cases, in fact, the impacts of acid rain on trees are due to the combined effects of acid rain and these other environmental stressors.

Nature is not the only area harmed by acid rain. Acid rain and the dry deposition of acidic particles contribute to the corrosion of metals (such as bronze). The damage that acid rain does to limestone and marble buildings and sculptures is due to a classic acid–base reaction. Marble and limestone both consist of calcium carbonate (CaCO3), a salt derived from the weak acid H2CO3. The reaction of a strong acid with a salt of a weak acid goes to completion. Thus we can write the reaction of limestone or marble with dilute sulfuric acid as follows:
\[{\mathrm{CaCO}}_{3(s)}\;+\:{\mathrm H}_2{\mathrm{SO}}_{4(aq)}\:\rightarrow\;{\mathrm{CaSO}}_{4(s)}\;+\:{\mathrm H}_2{\mathrm O}_{(l)}\:+\:{\mathrm{CO}}_{2(g)}\]
Because CaSO4 is sparingly soluble in water, the net result of this reaction is to dissolve the marble or limestone. These effects significantly reduce the societal value of buildings, bridges, and cultural objects such as statues, monuments, and tombstones (Figure \(\PageIndex{3}\)).

Sulfates and nitrates that form in the atmosphere from sulfur dioxide (SO2) and nitrogen oxide (NOx) emissions contribute to visibility impairment, meaning we cannot see as far or as clearly through the air. The pollutants that cause acid rain—SO2 and NOx—damage human health. These gases interact in the atmosphere to form fine sulfate and nitrate particles that can be transported long distances by winds and inhaled deep into people’s lungs. Fine particles can also penetrate indoors. Many scientific studies have identified a relationship between elevated levels of fine particles and increased illness and premature death from heart and lung disorders, such as asthma and bronchitis.
Acid Rain Program
In an effort to deal with the effects of acid rain, the Acid Rain Program (ARP) was established in 1995 as part of the Clean Air Act. It required major emission reductions of SO2 and NOx from the power sector. For SO2, a permanent cap was set at 8.95 million tons, about one-half of the emissions from the power sector in 1980. NOx reductions were achieved via a more traditional, rate-based regulatory system. Since the program began, the ARP has achieved significant emission reductions for both SO2 and NOx as seen in Figure \(\PageIndex{4}\).
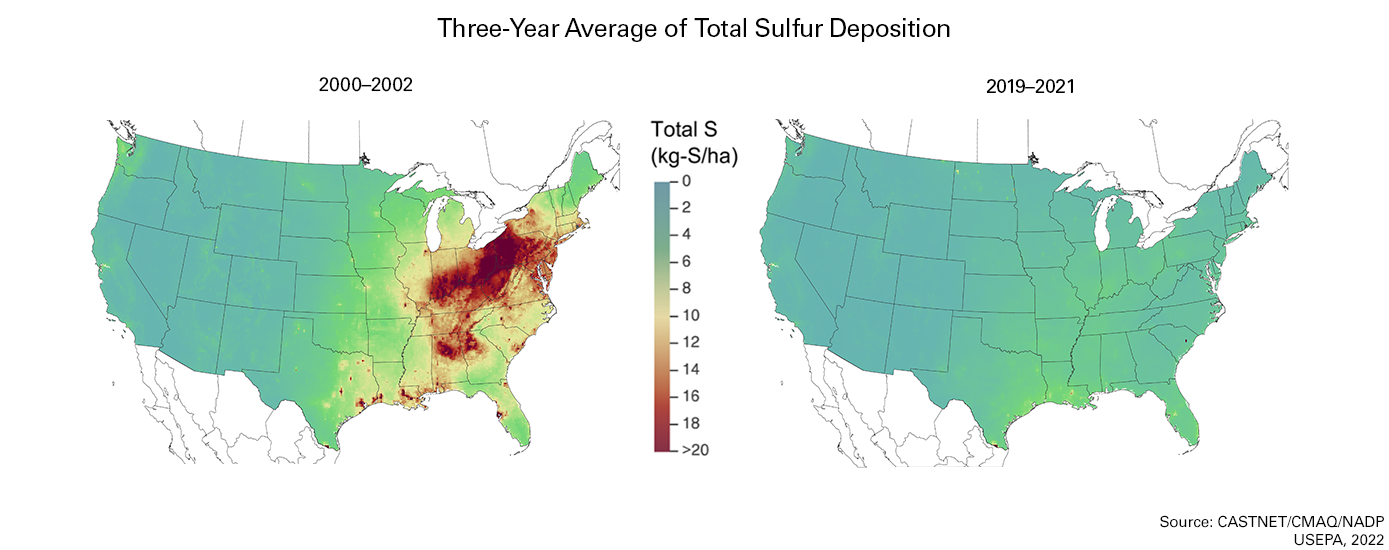

The ARP was the first national cap and trade program in the country and it introduced a system of allowance trading that uses market-based incentives to reduce pollution. This provides regulated sources with the flexibility to select the most cost-effective approach to reduce emissions and has proven to be a highly effective way to achieve emission reductions and thus attain environmental goals and improve human health.
Some measures can also be taken to mitigate the effects of acid rain, although these are very limited once the pollutant has formed. Some success has been achieved with treating acidified lakes with pulverized limestone to neutralize acid. Corrosion-resistant materials can be used in applications where exposure to acid rain is likely. Protective coatings, such as corrosion-resisting paint primers on metals, can be applied to materials likely to be exposed to acidic precipitation. But the best protection is to prevent formation and release of SO2 and NOx gases leading to acid rain formation by measures described earlier in this chapter.
Summary
- Acid rain is a term referring to a mixture of wet and dry deposition (deposited material) from the atmosphere containing higher than normal amounts of nitric and sulfuric acids.
- Acidic rainwater in the soil, streams, lakes, and marshes (and other bodies of water) can be harmful to trees, plants, and animals, especially aquatic plants and animals.
- Acid rain and the dry deposition of acidic particles contribute to the corrosion of metals (such as bronze) and the deterioration of paint and stone (such as marble and limestone).
Contributors and Attributions
- Essentials of Environmental Science by Kamala Doršner is licensed under CC BY 4.0. Modified from the original by Matthew R. Fisher.
- Charles Ophardt, Professor Emeritus, Elmhurst College
- Virtual Chembook
- TextMap: General Chemistry (Averill and Eldredge)
- US Environmental Protection Agency (EPA)
- Stanley E. Manahan (University of Missouri)
- Vicki MacMurdo (Anoka-Ramsey Community College)

