11.4: Oxidation and Reduction Reactions of Triglycerides
- Page ID
- 434349
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Describe the importance of key reactions of triglycerides such as oxidation and reduction of alkenes.
- Understand the origin and consequences of trans fats in our diet.
Triglycerides contain alkene and ester functional groups. In this section we will study two reactions based on the alkene functional group.
- Oxidation of alkenes
- Reduction of alkenes
Oxidation of Alkenes
When an akene is exposed to air, a slow oxidation reaction takes place. Atmospheric O2 is responsible for oxidation and breaking of the double bond. The breakdown products are aldehydes and carboxylic acids as shown in figure 11.4.1.

Figure \(\PageIndex{1}\): Oxidation of an alkene double bond to form aldehydes and carboxylic acids.
Triglycerides (fats and oils) that are in contact with moist air undergo oxidation of the alkene functional group and hydrolysis of esters. The breakdown products are small organic molecules that are aldehydes and carboxylic acids, that have unpleasant odors. When this happens, the triglyceride has spoiled or has gone rancid. Vegetable oils have a greater number of double bonds and so spoil more quickly compared to fats. See figure 11.4.2.

Figure \(\PageIndex{2}\): Oxygen in air can cause the oxidation of the alkenes in a triglyceride, producing small organic molecules that have unpleasant odors.
To make the oils last longer you can keep them refrigerated. The rate of the oxidation reaction is slower at lower temperatures. The oils may be stored in tightly capped bottles to lower the exposure to O2. Antioxidant molecules slow the oxidation of alkenes may also be added to the oils to increase shelf life. The oils are often stored in dark bottles to limit exposure to light. Removing some of the double bonds by partial hydrogenation also slows rancidity or spoilage.
Reduction of Alkenes or Catalytic Hydrogenation
Alkenes react with H2 in presence of a catalyst to alkanes as shown in figure 11.4.3.
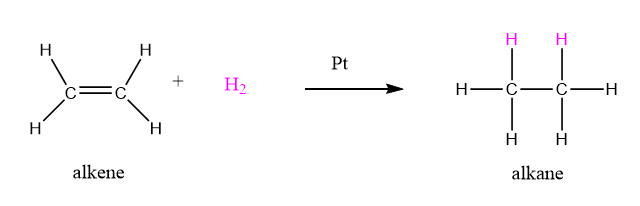
Figure \(\PageIndex{3}\): Reduction of an alkene to an alkane.
The alkene functional groups in unsaturated triglycerides can undergo the same catalytic reduction reaction of figure 11.4.3. Commercially during partial hydrogenation inexpensive and abundant vegetable oils (canola, corn, soybean) is treated with H2 and Pt, However the reaction is halted before all the double bonds are removed. Figure 11.4.4 shows the two double bonds in purple are hydrogenated. Partial hydrogenation converts vegetable oils into a semisolid product which has a good consistency for cooking but does not contain the cholesterol that is present in fats from animal sources. With fewer double bonds the partially hydrogenated vegetable oils do not oxidize (spoil) as rapidly.
 x
x
Figure \(\PageIndex{4}\): Partial Reduction of a triglyceride.
Trans fats
During the partial hydrogenation of vegetable oils an isomerization reaction occurs which converts a cis-double bond of a triglyceride to a trans-double bond as shown in figure 11.4.5.

Figure \(\PageIndex{5}\): Cis to trans isomer formation during partial hydrogenation.
A cis-double bond in a fatty acid introduces a kink in the structure. This kink or bend in the hydrocarbon part does not let the molecules pack with a large contact area between neighboring molecules. The London Forces are weaker in a triglyceride with cis-double bonds. Trans-fatty acids do not have a bend in the structure, and thus pack closely together in the same way that the saturated fats do as shown in figure 11.4.6.

Figure \(\PageIndex{6}\): Comparison of a trans fatty acid with a saturated and a cis fatty acid.
Studies have shown that trans fats raise cholesterol levels and increase the incidence of heart disease. They raise your bad (LDL) cholesterol levels and lower your good (HDL) cholesterol levels. Eating trans fats increases your risk of developing heart disease and stroke. It’s also associated with a higher risk of developing type 2 diabetes.
Naturally-occurring trans fats are produced in the gut of some animals and foods made from these animals (e.g., milk and meat products) may contain small quantities of these fats.
Summary
The oxidation of the alkene functional group in a triglyceride can form compounds with a disagreeable odor. Double bonds present in unsaturated triglycerides can be hydrogenated to convert oils to a semisolid product like margarine. During the process of partial hydrogenation some of the cis double bonds may be isomerized to trans.


