11.5: Neutralization of Fatty Acids and Hydrolysis of Triglycerides
- Page ID
- 434427
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Describe how carboxylic acids react with basic compounds.
- Identify the products of an acidic or basic hydrolysis of an ester.
- Understand the interactions of soap and detergent with hard water.
Neutralization Reaction of a Carboxylic Acid with NaOH
Carboxylic acids react with aqueous solutions of sodium hydroxide (NaOH) to form salts and water in a double replacement reaction. This reaction is an acid-base reaction known as neutralization reaction.
CH3COOH + NaOH(aq) → CH3COO−Na+(aq) + H2O
The salt is the conjugate base of the carboxylic acid is called the carboxylate ion (CH3COO−Na+).
Carboxylate ions (RCOO−Na+) are more soluble in water than carboxylic acids (RCOOH).
Neutralization Reaction of a Fatty Acid with NaOH
Fatty acids are long chain carboxylic acids. They are insoluble in water and hence hydrophobic. When treated with NaOH, fatty acids are converted into carboxylate ion salts (ionic compounds) containing Na+ and amphipathic fatty acid anion.

Figure \(\PageIndex{1}\): Oleic acid (hydrophobic), when reacted with aq NaOH, it is converted to an ionic compound sodium oleate (amphipathic)
Like all fatty acids, oleic acid is hydrophobic. In figure 11.5.1 oleic acid is converted into sodium oleate. Sodium oleate is soap. In aqueous solutions, soaps will spontaneously form micelles, a spherical structure that allows the hydrophobic tails to avoid contact with water and simultaneously form cluster in the center via London dispersion forces. The hydrophilic heads make up the outer surface and dissolves in water.

Figure \(\PageIndex{2}\): Micelle
Dirt and grime usually adhere to skin and clothing by combining with body oils, cooking fats, and lubricating greases. These substances are nonpolar and so they are not miscible in water. Washing with water alone will not remove them. These nonpolar oils and grease along with dirt will be trapped in the hydrophobic interior of the micelle of soap, while the micelle is easily rinsed away with water.
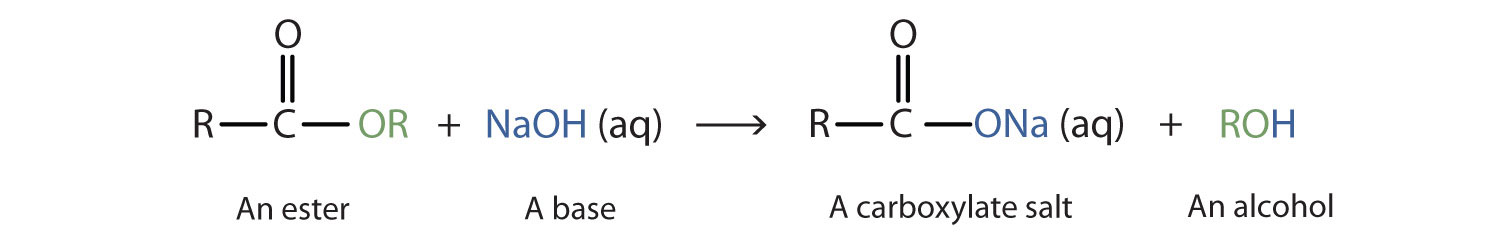
Hydrolysis of Esters
Hydrolysis is “splitting with water.” Hydrolysis is the most important reaction of esters. The hydrolysis of esters is catalyzed by either an acid or a base. During acid catalysed hydrolysis the ester is heated with water containing a small amount of a strong-acid catalyst such as sulfuric acid. The sulfuric acid is a source of the hydrogen ion (H+), the catalyst in the reaction.
Acidic hydrolysis of an ester gives a carboxylic acid and an alcohol. Note that the -OH of the carboxylic acid and the H of the alcohol comes from water (shown in purple).

When a base (such as sodium hydroxide [NaOH] or potassium hydroxide [KOH]) is used to hydrolyze an ester, the products are a carboxylate salt and an alcohol.
As a specific example, ethyl acetate and NaOH react to form sodium acetate and ethanol:
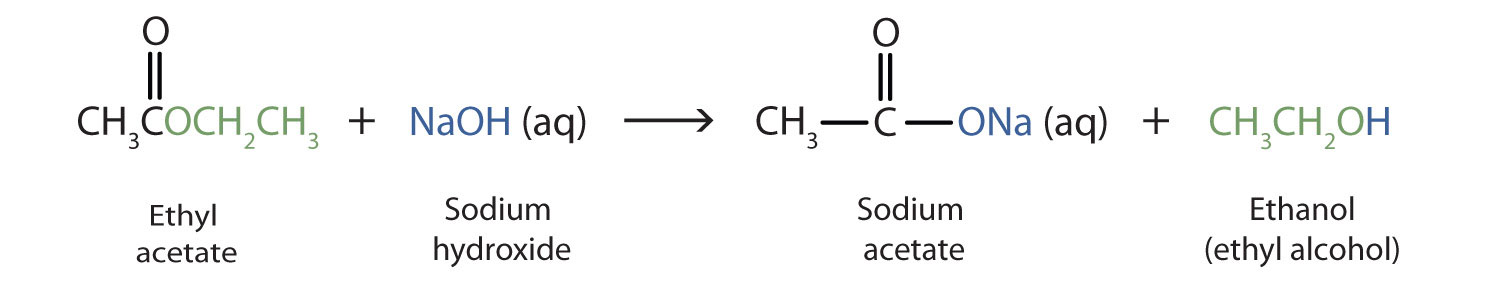
Because soaps are prepared by the alkaline hydrolysis of fats and oils, alkaline hydrolysis of esters is called saponification (Latin sapon, meaning “soap,” and facere, meaning “to make”). In a saponification reaction, the base is a reactant and a catalyst.
Saponification of Fats and Oils
Fats and oils are esters, they can be hydrolyzed in the presence of an acid, a base, or specific enzymes known as lipases. The hydrolysis of fats and oils in the presence of a base is used to make soap and is called saponification. Soaps are prepared through the hydrolysis of triglycerides (often from tallow, coconut oil, or both) using water and sodium hydroxide.

Figure \(\PageIndex{3}\): Saponification of a triglyceride.
Sodium salts of fatty acids make hard soaps while potassium salts make soft soaps. Three of the most common soaps are sodium stearate, sodium oleate, and sodium linoleate. Salts of saturated fatty acids make hard soap and those of polyunsaturated fatty acids make soft soap. Perfumes are added for scent and dyes for color, sand is added in scouring soap, and the air is blown into the soap to make it float on water.
Looking Closer: Soaps
Ordinary soap is a mixture of the sodium salts of various fatty acids, produced in one of the oldest organic syntheses practiced by humans (second only to the fermentation of sugars to produce ethyl alcohol). Both the Phoenicians (600 BCE) and the Romans made soap from animal fat and wood ash. Even so, the widespread production of soap did not begin until the 1700s. Soap was traditionally made by treating molten lard or tallow with a slight excess of alkali in large open vats. The mixture was heated, and steam was bubbled through it. After saponification was completed, the soap was precipitated from the mixture by the addition of sodium chloride (NaCl), removed by filtration, and washed several times with water. It was then dissolved in water and reprecipitated by the addition of more NaCl. The glycerol produced in the reaction was also recovered from the aqueous wash solutions.
Pumice or sand is added to produce scouring soap, while ingredients such as perfumes or dyes are added to produce fragrant, colored soaps. Blowing air through molten soap produces a floating soap. Soft soaps, made with potassium salts, are more expensive but produce a finer lather and are more soluble. They are used in liquid soaps, shampoos, and shaving creams.

Figure \(\PageIndex{4}\): Micelle of Soap.
Dirt and grime usually adhere to skin, clothing, and other surfaces by combining with body oils, cooking fats, lubricating greases, and similar substances that act like glues. Because these substances are not miscible in water, washing with water alone does little to remove them. Soap removes them, because soap molecules have a dual nature. One end, called the head, carries an ionic charge (a carboxylate anion) and therefore dissolves in water; the other end, the tail, has a hydrocarbon structure and dissolves in oils. The hydrocarbon tails dissolve in the soil; the ionic heads remain in the aqueous phase. With the oil no longer “gluing” the dirt to the soiled surface (skin, cloth, dish), the soap-enclosed dirt can easily be rinsed away.
Hard water
Water is considered hard water, if it contains soluble Ca2+, Mg2+ and Fe3+ ions. These ions are present in water as soluble bicarbonate compounds. If these ions are removed or absent, the water is considered soft water. Hard water can be purified by using water softeners or ion-exchange resins that replace Ca2+ and Mg2+ with Na+ ions.
Soaps
A soap is the sodium or potassium salt of a fatty acid. The tail part of the soap molecule is nonpolar, but the polar “head” at the carboxyl end permits a small portion of the molecule to be water soluble. Hence soaps are amphipathic. The cleansing power of soap is dependent on this water solubility. However, hard water containing Ca2+ and Mg2+ reacts with soap and forms insoluble scales (scum) with soap. Figure 11.5.5 shows the formation of insoluble precipitate of soap with Ca2+ ion in hard water. This reduces the cleansing properties of soap since less soap molecules are available to form the micelle.
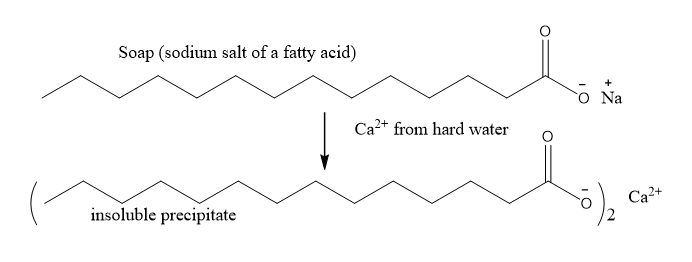
Figure \(\PageIndex{5}\): Formation of insoluble scales of soap with calcium ions in hard water.
Detergents
A detergent is the sodium salt of a sulfonic acid. It has similar cleansing properties to soap since detergents can form micelles just like soap. However, detergents differ from soaps in its interactions with hard water. The calcium and magnesium ions from hard water interact with a detergent and from compounds that are soluble in water. Figure 11.5.6 shows the formation of a soluble compound of detergent with Ca2+ ion in hard water. So, calcium and magnesium ions from hard water have no effect on the cleansing properties of a detergent.

Figure \(\PageIndex{6}\): Formation of soluble compound of detergent with calcium ions in hard water.
Summary
Fatty acids can react with bases to form an amphipathic compound called soap. Soaps are made by the hydrolysis reaction of a triglyceride with an aqueous solution of a base.


