11.3: Triglycerides- Fats and Oils
- Page ID
- 434084
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Explain why fats and oils are referred to as triglycerides.
- Explain how the fatty acid composition of the triglycerides determines whether a substance is a fat or oil.
Fats and oils are the most abundant lipids in nature. They provide energy for living organisms, insulate body organs, and transport fat-soluble vitamins through the blood.
Structures of Triglycerides
Fats and oils are called triglycerides (or triacylglycerols) because they are esters composed of three fatty acid units joined to glycerol, a trihydroxy alcohol:
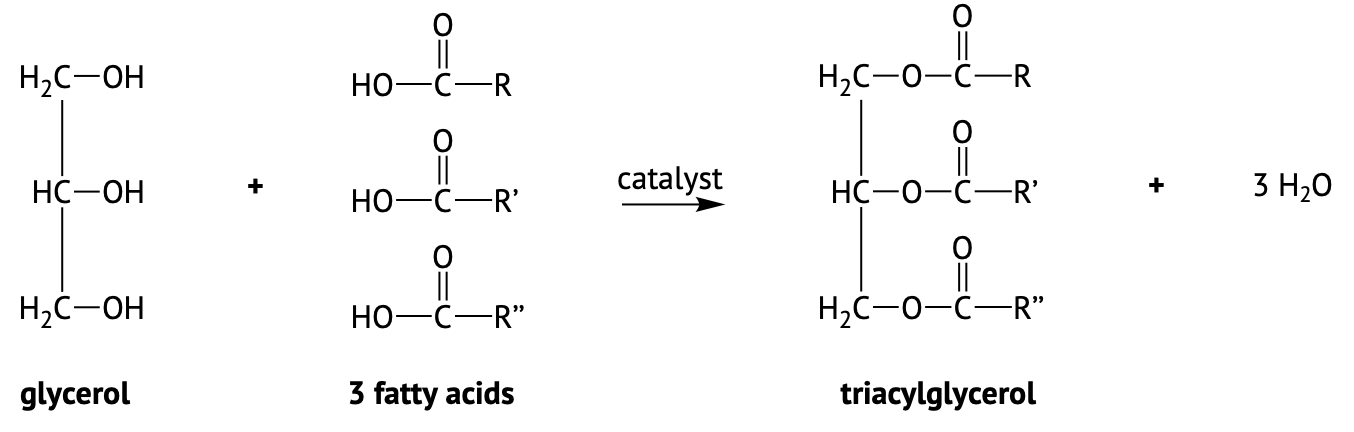
If all three OH groups on the glycerol molecule are esterified with the same fatty acid, the resulting ester is called a simple triglyceride. Although simple triglycerides have been synthesized in the laboratory, they rarely occur in nature. Instead, a typical triglyceride obtained from naturally occurring fats and oils contains two or three different fatty acid components and is thus termed a mixed triglyceride.
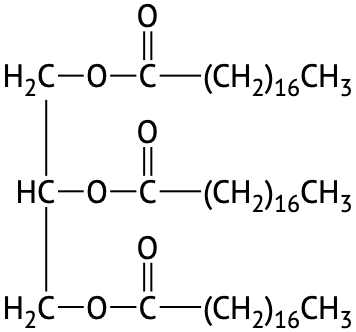
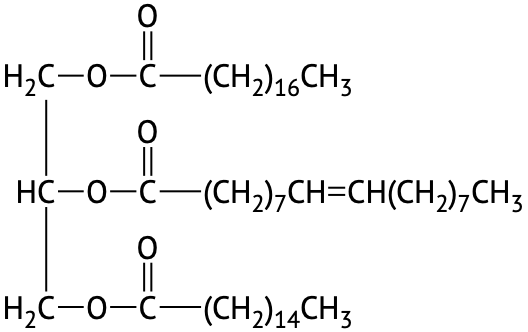
A triglyceride is called a fat if it is a solid at 25°C; it is called an oil if it is a liquid at that temperature. These differences in melting points reflect differences in the number of double bonds and number of carbon atoms in the constituent fatty acids. Triglycerides obtained from animal sources are usually fats, while those of plant origin are generally oils. Therefore, we commonly speak of animal fats and vegetable oils.
No single formula can be written to represent the naturally occurring fats and oils because they are highly complex mixtures of triglycerides in which many different fatty acids are represented. Table \(\PageIndex{1}\) shows the fatty acid compositions of some common fats and oils. The composition of any given fat or oil can vary depending on the plant or animal species it comes from, as well as on diet and climatic factors. To cite just one example, lard from corn-fed hogs is more highly saturated than lard from peanut-fed hogs. Palmitic acid is the most abundant of the saturated fatty acids, while oleic acid is the most abundant unsaturated fatty acid.
| Lauric | Myristic | Palmitic | Stearic | Oleic | Linoleic | Linolenic | |
|---|---|---|---|---|---|---|---|
| Fats | |||||||
| butter (cow) | 3 | 11 | 27 | 12 | 29 | 2 | 1 |
| tallow | 3 | 24 | 19 | 43 | 3 | 1 | |
| lard | 2 | 26 | 14 | 44 | 10 | ||
| Oils | |||||||
| canola oil | 4 | 2 | 62 | 22 | 10 | ||
| coconut oil† | 47 | 18 | 9 | 3 | 6 | 2 | |
| corn oil | 11 | 2 | 28 | 58 | 1 | ||
| olive oil | 13 | 3 | 71 | 10 | 1 | ||
| peanut oil | 11 | 2 | 48 | 32 | |||
| soybean oil | 11 | 4 | 24 | 54 | 7 | ||
| *Totals less than 100% indicate the presence of fatty acids with fewer than 12 carbon atoms or more than 18 carbon atoms. | |||||||
| †Coconut oil is highly saturated. It contains an unusually high percentage of the low-melting C8, C10, and C12 saturated fatty acids. | |||||||
Terms such as saturated fat or unsaturated oil are often used to describe the fats or oils obtained from foods. Saturated fats contain a high proportion of saturated fatty acids, while unsaturated oils contain a high proportion of unsaturated fatty acids. The high consumption of saturated fats is a factor, along with the high consumption of cholesterol, in increased risks of heart disease.
Biological Roles of Triglycerides
One of the primary biological roles of triglycerides is to provide energy. Gram for gram, they pack more than twice the caloric content of carbohydrates and proteins. The oxidation of fats and oils supplies about 9 kcal of energy for every gram oxidized, whereas the oxidation of carbohydrates or proteins supplies 4 kcal/g. Although the high caloric content of fats may be bad news for the dieter, it says something about the efficiency of nature’s designs. Our bodies use carbohydrates, primarily in the form of glucose, for our immediate energy needs. Our capacity for storing carbohydrates for later use is limited to tucking away a bit of glycogen in the liver or in muscle tissue. We store our reserve energy in lipid form. Triglycerides have other biological functions besides energy storage. They serve as protective padding and insulation for vital organs. The layers of fats in seals, penguins, and other aquatic animals provide thermal insulation and bouyancy in water. Furthermore, without lipids in our diets, we would be deficient in the fat-soluble vitamins A, D, E, and K since they transport fat-soluble vitamins through the blood..
Physical Properties of Fats and Oils
Contrary to what you might expect, pure fats and oils are colorless, odorless, and tasteless. The characteristic colors, odors, and flavors that we associate with some of them are imparted by foreign substances that are lipid soluble and have been absorbed by these lipids. For example, the yellow color of butter is due to the presence of the pigment carotene; the taste of butter comes from two compounds—diacetyl and 3-hydroxy-2-butanone—produced by bacteria in the ripening cream from which the butter is made.
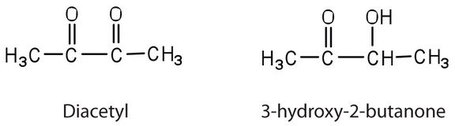
Fats and oils are lighter than water, having densities of about 0.8 g/cm3. They are poor conductors of heat and electricity and therefore serve as excellent insulators for the body, slowing the loss of heat through the skin. Since they contain long carbon chains, triglycerides are nearly nonpolar molecules and thus do not dissolve readily in polar solvents such as water. Instead oils and fats are soluble in nonpolar organic solvents composed of alkanes such as hexane (CH3CH2CH2CH2CH2CH3) or octane (CH3CH2CH2CH2CH2CH2CH2CH3).
Summary
Fats and oils are molecules known as triglycerides, which are esters of three fatty acids linked to glycerol. An increase in the percentage of shorter-chain fatty acids and/or unsaturated fatty acids lowers the melting point of a triglyceride.


