Chemistry of Titanium
- Page ID
- 639
Discovered independently by William Gregor and Martin Klaproth in 1795, titanium (named for the mythological Greek Titans) was first isolated in 1910. Gregor, a Cornish vicar and amateur chemist isolated an impure oxide from ilmenite (\(FeTiO_3\)) by treatment with \(HCl\) and \(H_2SO_4\).Titanium is the second most abundant transition metal on Earth (6320 ppm) and plays a vital role as a material of construction because of its:
- Excellent Corrosion Resistance
- High Heat Transfer Efficiency
- Superior Strength-To-Weight Ratio
For example, when it's alloyed with 6% aluminum and 4% vanadium, titanium has half the weight of steel and up to four times the strength.
Uses of titanium
Titanium is a highly corrosion-resistant metal with great tensile strength. It is ninth in abundance for elements in the earth's crust. It has a relatively low density (about 60% that of iron). It is also the tenth most commonly occurring element in the Earth's crust. That all means that titanium should be a really important metal for all sorts of engineering applications. In fact, it is very expensive and only used for rather specialized purposes. Titanium is used, for example:
- in the aerospace industry - for example in aircraft engines and air frames;
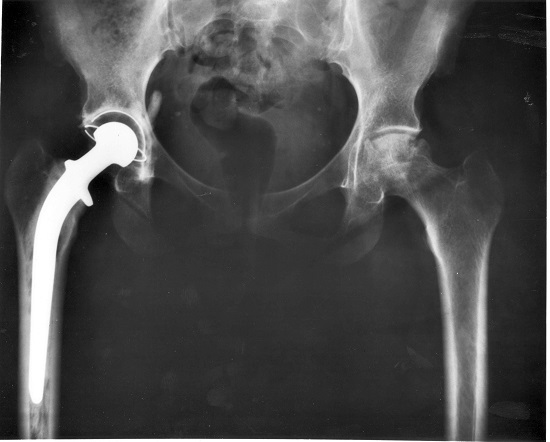
- for replacement hip joints;
- for pipes, etc, in the nuclear, oil and chemical industries where corrosion is likely to occur.
Titanium is very expensive because it is awkward to extract from its ores - for example, from rutile, \(TiO_2\). Whilst a biological function in man is not known, it has excellent biocompatibility--that is the ability to be ignored by the human body's immune system--and an extreme resistance to corrosion. Titanium is now the metal of choice for hip and knee replacements.
Titanium Extraction
Titanium cannot be extracted by reducing the ore using carbon as a cheap reducing agent, like with iron. The problem is that titanium forms a carbide, \(\ce{TiC}\), if it is heated with carbon, so you don't get the pure metal that you need. The presence of the carbide makes the metal very brittle. That means that you have to use an alternative reducing agent. In the case of titanium, the reducing agent is either sodium or magnesium. Both of these would, of course, first have to be extracted from their ores by expensive processes.
The titanium is produced by reacting titanium(IV) chloride, \(\ce{TiCl4}\) - NOT the oxide - with either sodium or magnesium. That means that you first have to convert the oxide into the chloride. That in turn means that you have the expense of the chlorine as well as the energy costs of the conversion. High temperatures are needed in both stages of the reaction.
Titanium is made by a batch process. In the production of iron, for example, there is a continuous flow through the Blast Furnace. Iron ore and coke and limestone are added to the top, and iron and slag removed from the bottom. This is a very efficient way of making something. With titanium, however, you make it one batch at a time. Titanium(IV) chloride is heated with sodium or magnesium to produce titanium. The titanium is then separated from the waste products, and an entirely new reaction is set up in the same reactor. This is a slow and inefficient way of doing things. Traces of oxygen or nitrogen in the titanium tend to make the metal brittle. The reduction has to be carried out in an inert argon atmosphere rather than in air; that also adds to costs.
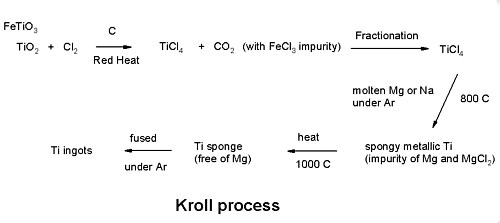
Wilhelm J. Kroll developed the process in Luxemburg around the mid 1930's and then after moving to the USA extended it to enable the extraction of Zirconium as well. Titanium ores, mainly rutile (\(\ce{TiO2}\)) and ilmentite (\(\ce{FeTiO3}\)), are treated with carbon and chlorine gas to produce titanium tetrachloride.
\[\ce{TiO2 + Cl2 \rightarrow TiCl4 + CO2} \nonumber \]
Fractionation
Titanium tetrachloride is purified by distillation (Boiling point of 136.4) to remove iron chloride.
Reduction
Purified titanium tetrachloride is reacted with molten magnesium under argon to produce a porous “titanium sponge”.
\[\ce{TiCl4 + 2Mg \rightarrow Ti + 2MgCl2} \nonumber \]
Melting
Titanium sponge is melted under argon to produce ingots.
Titanium Halides
| Formula | Color | MP | BP | Structure |
|---|---|---|---|---|
| TiF4 | white | - | 284 | fluoride bridged |
| TiCl4 | Colorless | -24 | 136.4 | - |
| TiBr4 | yellow | 38 | 233.5 | hcp I- but essentially monomeric cf. SnI4 |
| TiI4 | violet-black | 155 | 377 | hcp I- but essentially monomeric cf. SnI4 |
Preparations
They can all be prepared by direct reaction of Ti with halogen gas (X2). All are readily hydrolyzed. They are all expected to be diamagnetic.
| Formula | Color | MP | BP | m (BM) | Structure |
|---|---|---|---|---|---|
| TiF3 | blue | 950d | - | 1.75 | - |
| TiCl3 | violet | 450d | - | - | BiI3 |
| TiBr3 | violet | - | - | - | BiI3 |
| TiI3 | violet-black | - | - | - | - |
Preparations:
They can be prepared by reduction of TiX4 with H2.
Titanium Oxides and Aqueous Chemistry
| Formula | Color | MP | m (BM) | Structure |
|---|---|---|---|---|
| TiO2 | white | 1892 | diam. | rutile - Refractive Index 2.61-2.90 cf. Diamond 2.42 |
Preparations
obtained from hydrolysis of TiX4 or Ti(III) salts.
TiO2 reacts with acids and bases.
In Acid: TiOSO4 formed in H2SO4 (Titanyl sulfate)
In Base: MTiO3 metatitanates (eg Perovskite, CaTiO3 and ilmenite, FeTiO3) M2TiO4 orthotitanates.
Peroxides are highly colored and can be used for Colorimetric analysis.
pH <1 [TiO2(OH)(H2O)x]+
pH 1-2 [(O2)Ti-O-Ti(O2)](OH) x2-x; x=1-6
[Ti(H2O)6]3+ -> [Ti(OH)(H2O)5]2+ + [H+] pK=1.4
TiO2+ + 2H+ + e- -> Ti3+ + H2O E=0.1V
Representative complexes
TiCl4 is a good Lewis acid and forms adducts on reaction with Lewis bases such as;
2PEt3 -> TiCl4(PEt3)2
2MeCN -> TiCl4(MeCN)2
bipy -> TiCl4(bipy)
Solvolysis can occur if ionisable protons are present in the ligand;
2NH3 -> TiCl2(NH2)2 + 2HCl
4H2O -> TiO2.aq + 4HCl
2EtOH -> TiCl2(OEt)2 + 2HCl
TiCl3 has less Lewis acid strength but can form adducts also;
3pyr -> TiCl3pyr3
Conversion of titanium oxide into titanium chloride
The ore rutile (impure titanium(IV) oxide) is heated with chlorine and coke at a temperature of about 900°C.
\[ TiO_2 + 2Cl_2 + 2C \longrightarrow TCl_4 + 2CO \nonumber \]
Other metal chlorides are formed as well because of other metal compounds in the ore. Very pure liquid titanium(IV) chloride can be separated from the other chlorides by fractional distillation under an argon or nitrogen atmosphere. Titanium(IV) chloride reacts violently with water. Handling it therefore needs care and is stored in totally dry tanks.
Reduction of the titanium chloride
Reduction by sodium: The titanium(IV) chloride is added to a reactor in which very pure sodium has been heated to about 550°C - everything being under an inert argon atmosphere. During the reaction, the temperature increases to about 1000°C.
\[ \ce{TiCl4 +4Na \longrightarrow Ti + 4NaCl } \nonumber \]
After the reaction is complete, and everything has cooled (several days in total - an obvious inefficiency of the batch process), the mixture is crushed and washed with dilute hydrochloric acid to remove the sodium chloride.
Reduction by magnesium
This is the method used in the rest of the world. The method is similar to using sodium, but this time the reaction is:
\[ \ce{TiCl4 +4Mg \longrightarrow Ti + 2MgCl2 } \nonumber \]
The magnesium chloride is removed from the titanium by distillation under very low pressure at a high temperature.
Outside Links
- Beattie, James K. "A New Perspective on Rutile." J. Chem. Educ. 1998 75 641.
- Gisser, Kathleen R. C.; Geselbracht, Margaret; Cappellari, Ann; Hunsberger, Lynn; Ellis, Arthur B.; Perepezko, John; Lisensky, George C. "Nickel-Titanium Memory Metal: A "Smart" Material Exhibiting a Solid-State Phase Change and Superelasticity." J. Chem. Educ. 1994, 71, 334.
- "Complexes and First-Row Transition Elements", D. Nicholls
- "Basic Inorganic Chemistry", F.A. Cotton, G. Wilkinson and P.L. Gaus
- "Advanced Inorganic Chemistry", F.A. Cotton, G. Wilkinson, C. A. Murillo, and M. Bochmann
- "Chemistry of the Elements", Greenwood and Earnshaw
Contributors and Attributions
Jim Clark (Chemguide.co.uk)
- {{template.ContribLancashire()}}

