Hydrosilylation
- Page ID
- 69097
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Introduction
Hydrosilylation, also known as hydrosilation, is one of the most useful catalytic reactions leading to the formation of organsilanes and organosilicones, which have a variety of applications in industry and as intermediates in organic chemistry. Hydrosilylation occurs via the addition of H-Si to an unsaturated bond such as carbon-carbon bond, carbon-oxygen bond, carbon-nitrogen bond, nitrogen-nitrogen bond and nitrogen-oxygen bond using a metal catalyst, Lewis Acid, or radical initiator. The basic reaction can be described using the following scheme:
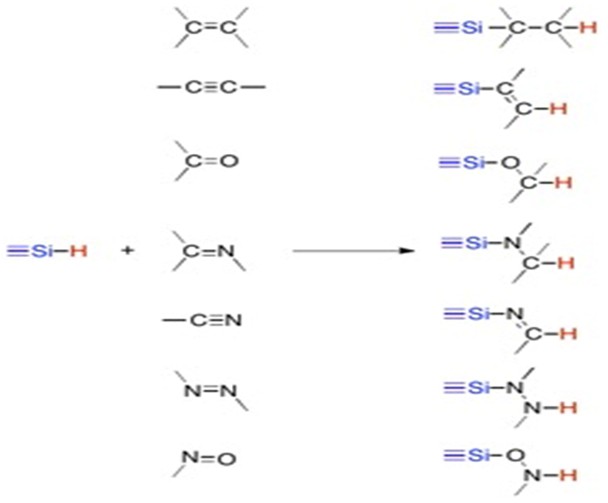
Mechanism of Metal Catalyzed Hydrosilanes
Hydrosilylation reaction is often completed with the association of a transition metal catalyst. In the first reported hydrosilylation reaction, the reaction was performed with trichlorosilane and 1-octene using crystalline diacetyl peroxide as the catalyst[3]. Due to the sensitivity and reactivity of diacetyl peroxide, safer metal catalysts are being used, the most common being platinum. Here is a typical catalyst cycle describing the mechanism:[4]
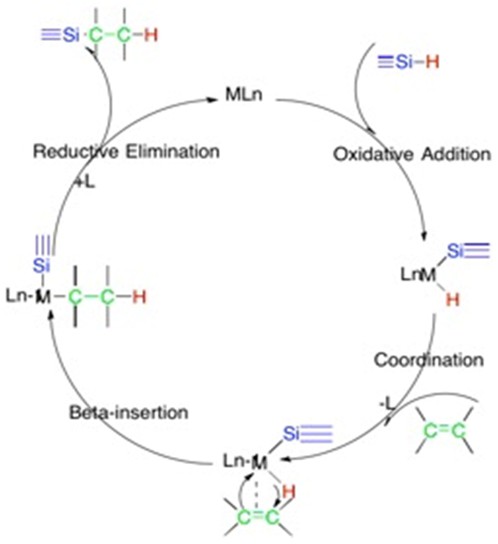
In the first step a Si-H bond undergoes oxidative addition to a metal center. The alkene coordinates and then undergoes insertion. The hydrogen is then added to the alkene through H beta-insertion. In the final step the alkylsilyl Pd(II) complex undergoes reductive elimination to deliver the adduct and return the metal to the original oxidation state.
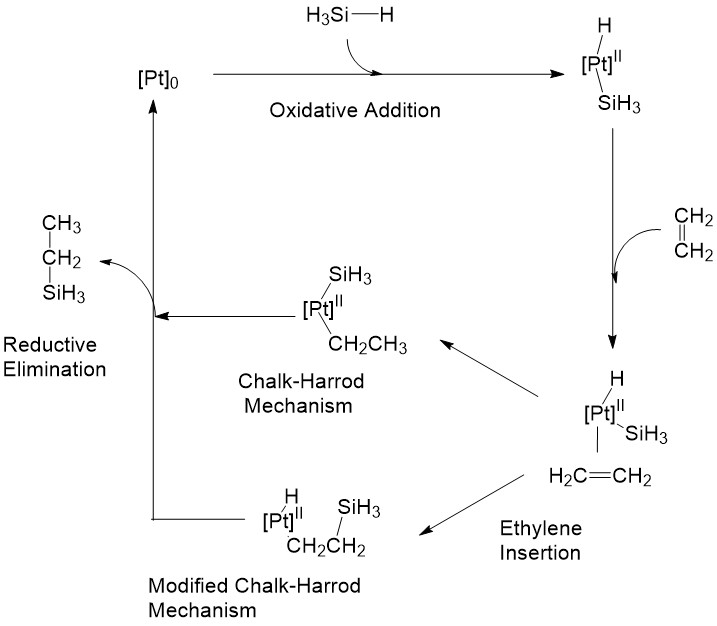
A well-known mechanism of metal catalyzed hydrosilylation is the Chalk-Harrod mechanism. It proceeds through oxidative addition of the Si-H to the metal center and then alkene insertion to the metal hydride. Reductive elimination of Si-C then occurs and gives the final product. A modified version of the Chalk-Harrod method was also discovered where the reaction proceeds through an ethylene insertion into the metal silyl bond. Then reductive elimination of C-H occurs to give the vinylsilane product.[5]
Reactivity
The reactivity of hydrosilylation is influenced by many factors: substrate, silane, transition metal catalyst, ligand etc. which makes the reaction diverse as well as complicated. Studies have been performed to determine the reactivity influenced by each of these factors. Because there are a large variety of substrates that can be used, we will focus on alkenes. Here are some general rules:
Substrate Group: Alkene
Reaction rate: 1-alkene > 2-alkene > 3-alkene
Generally, the more substituted the alkene group, the slower the reaction would be. This is due to the more substituted alkene group being bulkier, therefore it will be harder for the alkene coordination to happen to the metal. More substituted alkene groups also make it more difficult for the H atom to undergo compete beta-insertion.
Silyl Group
Reaction rate: SiHCl3 > (C6H5)3SiH > (C2H5)3SiH
V0(105): 110 12 1.2
From this set of data, we can derive that the rate of reactivity would increase depending on the substituted groups on the silanes. The order of reactivity is: chlorine > aromatic rings > alkanes. However, this rule is not always true. If we substituted the aromatic groups in (C6H5)3SiH with Cl, the reaction rate would be:
Reaction rate: (C6H5)3SiH > (C6H5)2SiH2 > (C6H5)SiH3
V0(105): 12 1.7 0.69
This shows that the substituted groups do not affect the reactivity individually. The overall regularity for change of silanes is complicated and varies with each group.
Transition Metal
The two-stage hydrosilyation of acetylene by trichlorosilane at 150oC in the gaseous phase using a flow method provided a test reaction for the catalytic activity of different transition metals. This reaction was supported by gamma-Al2O3 and an active carbon. Below is the yield order for the reaction:
Yield for gamma-Al2O3 supported reaction: Pd > Pt > Ru > Rh > Ir
Yield for active carbon supported reaction: Rh > Ir > Pd > Ru > Pt
Such a sequence could be used to help choosing catalyst based on different supporting materials.
Ligand Type
The metal catalyst is usually at an oxidation state of 0 or two oxidation states lower than what is the maximum for that metal. If there is complete saturation of the metal, then a ligand must be lost before the incorporation of the silane in the oxidative addition step. The ligands attached to the metal are usually made up of neutral and anionic ligands. Before coordination with the silane, the metal center must be unsaturated and therefore usually a ligand is lost prior to oxidative addition.
\[L_{n}M^{X+}\rightarrow L_{n-1}M^{X+}\frac{\rightarrow}{HSiR_{3}}(H)L_{n-1}M^{(X+2)}SiR_{3} \nonumber \]
L = neutral
\(L_{n-1}M^{X+}\)= oxidative metal
\((H)L_{n-1}M^{(X+2)}SiR_{3}\)= oxidative addition product
Common ligands used in TM complexes: CO and phosphines
Regio and Stereoselectivity
The stereo- and regioselectivity of hydrosilylation reactions are highly dependent on the substrate, catalyst, and solvent. There are almost always side products, and cocatalysts or directing groups are often necessary for regiocontrol. In the Chalk-Harrod mechanism the H-Si group is added across an alkene and the anti-markovnikov products are obtained. Hydrosilylation of terminal alkynes have three possible products whereas hydrosilylation of internal alkynes has four possible products:
Terminal Alkyne:
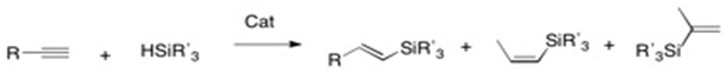
Internal Alkyne:
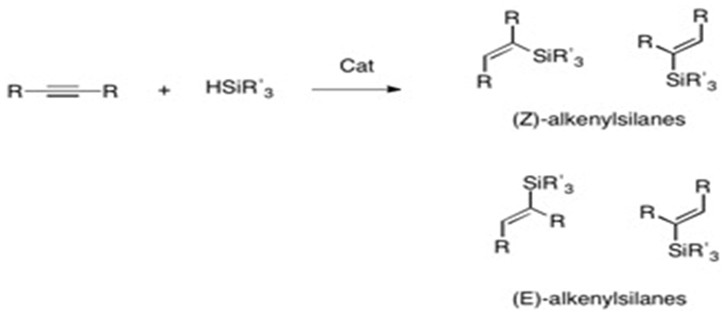
In terminal alkynes, cis- addition of hydrosilane gives the (E)-product while trans- addition gives the (Z)-product. An example of specific selectivity is in platinum catalized hydrosilylation: the reaction proceeds through cis-addition and produces α and β (E)-alkenylsilanes.
Other Methods of Hydrosilylation
Radical Initiation
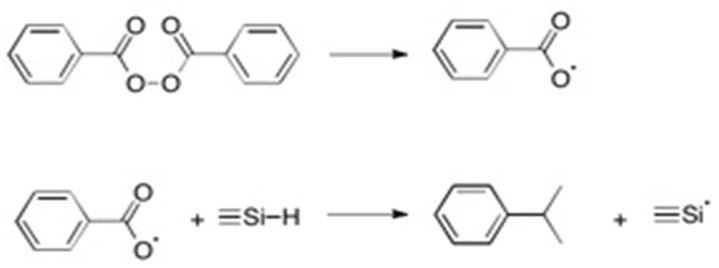
Another method of hydrosilylation is through a free radical mechanism. In this case, a radical initiator such as a superoxide is needed to provide a radical source. The mechanism can be described using two steps through the following scheme:[9]
Step 1:
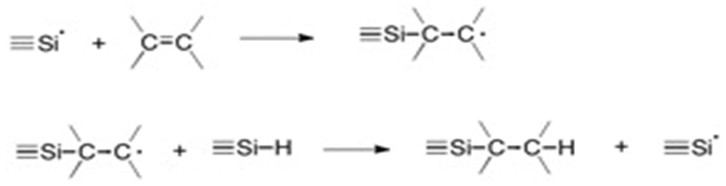
Step 2:
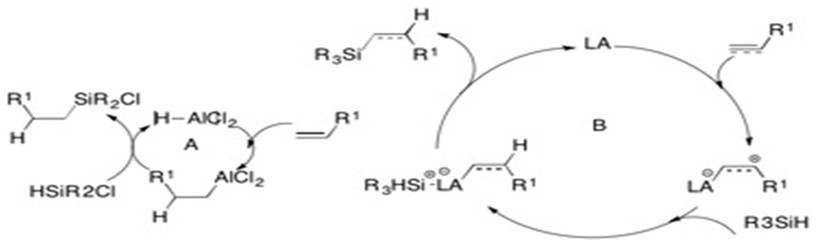
Lewis Acid
Hydrosilylation can also occur through the addition of an alkene or an alkyne to a silyl group and using an acid.[10]
References
[1] Kuhn, E Fritz, et al. Platinum Catalysis Revisited-Unraveling Principles of Catalytic Olefin Hydrosilylation. ACS Catalysis. 6 (2), pp 1274–1284
[2] Bogdan Marciniec. Comprehensive Handbook on Hydrosilylation. Elsevier, 2013. Print
[3] L.H Sommer, et al. Peroxide-Catalyzed Addition of Trichlorosilane to 1-Octene. J. Am. Chem. Soc., 1947, 69 (1), pp 188–188
[4] Bogdan Marciniec. Comprehensive Handbook on Hydrosilylation. Elsevier, 2013. Print
[5] Sakaki S. et al. Organometallics 1998, 17, 2510-2523
[6] Bogdan Marciniec. Comprehensive Handbook on Hydrosilylation. Elsevier, 2013. Print
[7] Corey, Joyce Y. “Reactions of Hydrosilanes with Transition Metal Complexes”. Chem. Rev., 2016, 116 (19), pp 11291–11435
[8] Marciniec, Bogdan. Hydrosilylation: A Comprehensive Review on Recent Advances. Advances in Silicon Science, Springer Science, 2009
[9] Bogdan Marciniec. Comprehensive Handbook on Hydrosilylation. Elsevier, 2013. Print
[10] Michael Rubin, Todd Schwier, Valdimir Gevorgyan*. Highly Efficient B(C6F5)3-Catalyzed Hydrosilylation of Olefins. J. Org. Chem. 2002, 67, 1936-1940
Contributors and Attributions
- Giovanna Romero and Xinyu (Wayne) Wang

