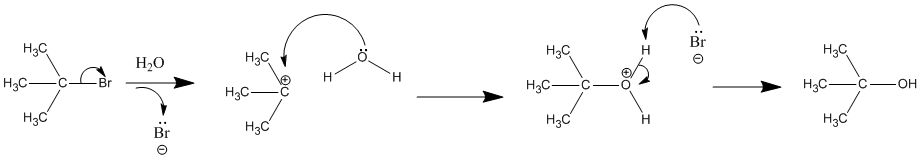
Effects of Solvent, Leaving Group, and Nucleophile on Unimolecular Substitution
- Page ID
- 1145
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Introduction
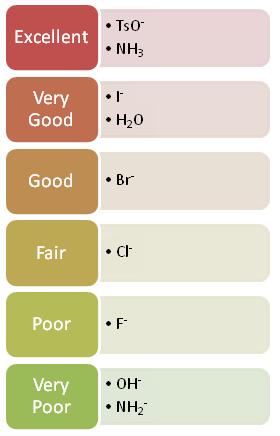
Since the hydrogen atom in a polar protic solvent is highly positively charged, it can interact with the anionic nucleophile which would negatively affect an SN2, but it does not affect an SN1 reaction because the nucleophile is not a part of the rate-determining step (See SN2 Nucleophile). Polar protic solvents actually speed up the rate of the unimolecular substitution reaction because the large dipole moment of the solvent helps to stabilize the transition state. The highly positive and highly negative parts interact with the substrate to lower the energy of the transition state. Since the carbocation is unstable, anything that can stabilize this even a little will speed up the reaction. Sometimes in an SN1 reaction the solvent acts as the nucleophile. This is called a solvolysis reaction (see example below). The polarity and the ability of the solvent to stabilize the intermediate carbocation is very important as shown by the relative rate data for the solvolysis (see table below). The dielectric constant of a solvent roughly provides a measure of the solvent's polarity. A dielectric constant below 15 is usually considered non-polar. Basically, the dielectric constant can be thought of as the solvent's ability to reduce the internal charge of the solvent. So for our purposes, the higher the dielectric constant the more polar the substance and in the case of SN1 reactions, the faster the rate.
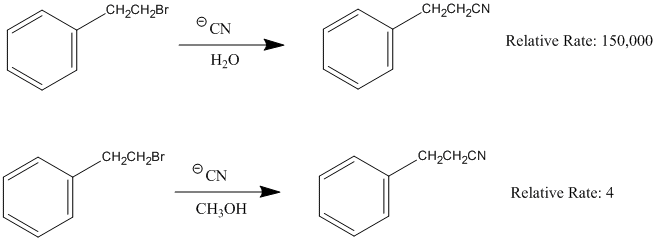
Effects of Nucleophile
The strength of the nucleophile does not affect the reaction rate of SN1 because, as stated above, the nucleophile is not involved in the rate-determining step. However, if you have more than one nucleophile competing to bond to the carbocation, the strengths and concentrations of those nucleophiles affects the distribution of products that you will get. For example, if you have (CH3)3CCl reacting in water and formic acid where the water and formic acid are competing nucleophiles, you will get two different products: (CH3)3COH and (CH3)33COCOH. The relative yields of these products depend on the concentrations and relative reactivities of the nucleophiles.Effects of Leaving Group
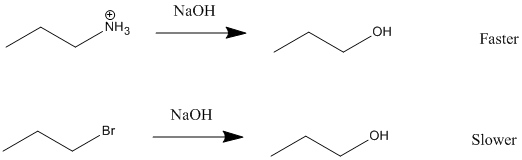
An SN1 reaction speeds up with a good leaving group. This is because the leaving group is involved in the rate-determining step. A good leaving group wants to leave so it breaks the C-Leaving Group bond faster. Once the bond breaks, the carbocation is formed and the faster the carbocation is formed, the faster the nucleophile can come in and the faster the reaction will be completed.
A good leaving group is a weak base because weak bases can hold the charge. They're happy to leave with both electrons and in order for the leaving group to leave, it needs to be able to accept electrons. Strong bases, on the other hand, donate electrons which is why they can't be good leaving groups. As you go from left to right on the periodic table, electron donating ability decreases and thus ability to be a good leaving group increases. Halides are an example of a good leaving group whos leaving-group ability increases as you go down the column.
.jpg?revision=1)
The two reactions below is the same reaction done with two different leaving groups. One is significantly faster than the other. This is because the better leaving group leaves faster and thus the reaction can proceed faster.
Other examples of good leaving groups are sulfur derivatives such as methyl sulfate ion and other sulfonate ions (See Figure below)
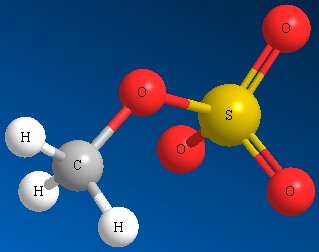
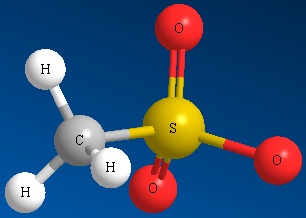
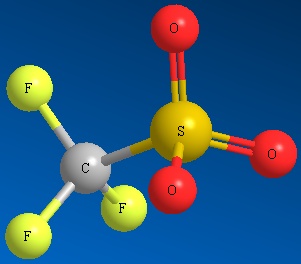
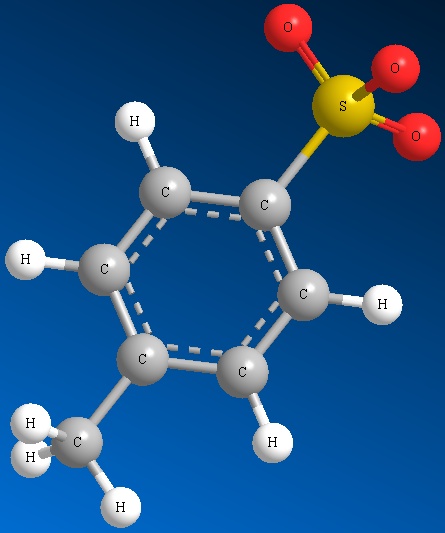
Methyl Sulfate Ion Mesylate Ion Triflate Ion Tosylate Ion
References
- Uggerud E. "Reactivity trends and stereospecificity in nucleophilic substitution reactions." J. Phys. Org. Chem. 2006; 19; 461-466.
- Vollhardt, K. Peter C., and Neil E. Schore. Organic Chemistry Structure and Function. New York: W. H. Freeman, 2007.
- Petrucci, Ralph H. General Chemistry: Principles and Modern Applications. Upper Saddle River, NJ: Pearson Education, 2007.
- Suggs, William J. Organic Chemistry. Canada: Barron's Educational Series Inc., 2002.
Outside Links
- http://en.Wikipedia.org/wiki/Sn1
- http://www.youtube.com/watch?v=FuFpjx_ZeT0 (Pretty good summary of SN1 reaction)
Problems
1. Put the following leaving groups in order of decreasing leaving group ability
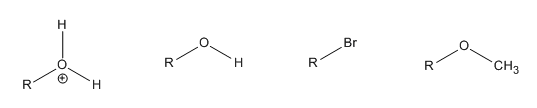
2. Which solvent would an SN1 reaction occur faster in? H2O or CH3CN
3. What kind of conditions disfavor SN1 reactions?
4. What are the products of the following reaction and does it proceed via SN1 or SN2?
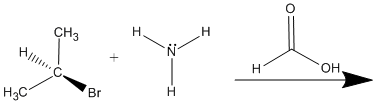
5. How could you change the reactants in the problem 4 to favor the other substitution reaction?
6. Indicate the expected product and list why it occurs through SN1 instead of SN2?
.gif?revision=1)
Answers
1. 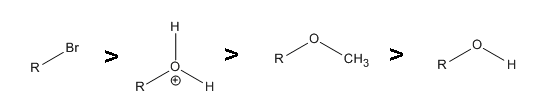.gif?revision=1)
2. An SN1 reaction would occur faster in H2O because it's polar protic and would stailize the carbocation and CH3CN is polar aprotic.
3. Polar aprotic solvents, a weak leaving group and primary substrates disfavor SN1 reactions.
4. 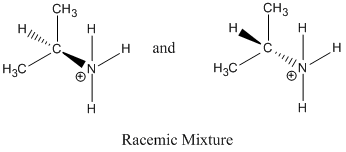
Reaction proceeds via SN1 because a tertiary carbocation was formed, the solvent is polar protic and Br- is a good leaving group.
5. You could change the solvent to something polar aprotic like CH3CN or DMSO and you could use a better base for a nucleophile such as NH2- or OH-.
6. 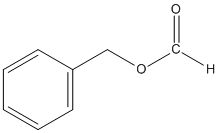
This reaction occurs via SN1 because Cl- is a good leaving group and the solvent is polar protic. This is an example of a solvolysis reaction because the nucleophile is also the solvent.
Contributors
- Ashiv Sharma