30.6: Polymer Structure and Physical Properties
- Page ID
- 364617
Polymer Crystallinity
To account for the physical differences between the different types of polymers, the nature of the aggregate macromolecular structure, or morphology, of each substance must be considered. Because polymer molecules are so large, they generally pack together in a non-uniform fashion, with ordered or crystalline-like regions, called crystallites, mixed together with disordered or amorphous domains. In some cases the entire solid may be amorphous, composed entirely of coiled and tangled macromolecular chains. Crystallinity occurs when linear polymer chains are structurally oriented in a uniform three-dimensional matrix. In the diagram on the right, crystalline domains are colored blue. Increased crystallinity is associated with an increase in rigidity, tensile strength and opacity (due to light scattering). Amorphous polymers are usually less rigid, weaker and more easily deformed. They are often transparent.
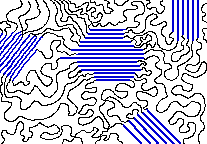
Three factors that influence the degree of crystallinity are:
- Chain length: Longer polymer chains tend to have greater van der Waals forces and increased crystallinity than do shorter chains (Section 2-12)
- Chain branching: Polymer chains without branching can pack closely together. Lack of branching increases the crystallinity of polymers.
- Interchain interactions: (Intermolecular forces and Crosslinking). Crystallinity increases as the strength of the intermolecular forces between polymer chains increases. Croslining between polymer chains tends to increase crystallinity.
The importance of the first two factors is nicely illustrated by the differences between LDPE and HDPE. As noted earlier (Section 31-2), HDPE is composed of very long unbranched hydrocarbon chains. These pack together easily in crystalline domains that alternate with amorphous segments, and the resulting material, while relatively strong and stiff, retains a degree of flexibility. In contrast, LDPE is composed of smaller and more highly branched chains which do not easily adopt crystalline structures. This material is therefore softer, weaker, less dense and more easily deformed than HDPE.
The forces between the chains in the crystallites of polyethene are the so-called van der Waals or dispersion forces, which are the same forces acting between smaller hydrocarbon molecules. Although these forces are relatively weak, they increase with the size of the molecule. Polymer chains are large enough that the van der Waals forces create a strong and stiff material.
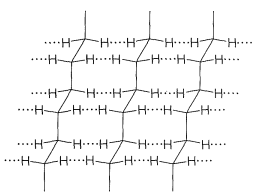
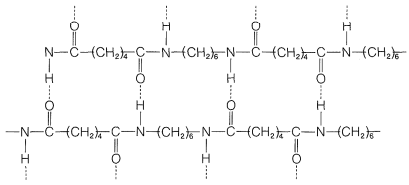
In other kinds of polymers, even stronger intermolecular forces can be produced by hydrogen bonding. This is especially important in the polyamides, such as the nylons, of which nylon 66 is most widely used. The increase strength of the interactions between polymer chains makes polyamides among some of the strongest polymer materials known.
Natural rubber is a completely amorphous polymer. Unfortunately, the potentially useful properties of raw latex rubber are limited by temperature dependence; however, these properties can be modified by chemical change. If the chains of rubber molecules are slightly cross-linked by sulfur atoms, a process called vulcanization which was discovered by Charles Goodyear in 1839, the desirable elastomeric properties of rubber are substantially improved. At 2 to 3% crosslinking a useful soft rubber, that no longer suffers stickiness and brittleness problems on heating and cooling, is obtained. At 25 to 35% crosslinking a rigid hard rubber product is formed. The following illustration shows a cross-linked section of amorphous rubber.
Glass Transition and Melt Transition Temperatures
The effect of temperature on the physical properties of polymers is very important to their practical uses. At low temperatures, polymers become hard and glasslike because the motions of the segments of the polymer chains with relation to each other are slow. The approximate temperature below which glasslike behavior is apparent is called the glass transition temperature and is symbolized by Tg. When a polymer containing crystallites is heated, the crystallites ultimately melt, and this temperature is usually called the melt transition temperature and is symbolized as Tm. As the amount of crystallinity in a polymer increases, so does Tm.
Tg often depends on the history of the sample, particularly previous heat treatment, mechanical manipulation and annealing. It is sometimes interpreted as the temperature above which significant portions of polymer chains are able to slide past each other in response to an applied force. The introduction of relatively large and stiff substituents (such as benzene rings) will interfere with this chain movement, thus increasing Tg (note polystyrene below). The introduction of small molecular compounds called plasticizers (discussed later in this section) into the polymer matrix increases the interchain spacing, allowing chain movement at lower temperatures. with a resulting decrease in Tg. Tm and Tg values for some common addition polymers are listed below.
| Polymer | LDPE | HDPE | PP | PVC | PS | PAN | PTFE | PMMA | Rubber |
|---|---|---|---|---|---|---|---|---|---|
| Tm (ºC) | 110 | 130 | 175 | 180 | 175 | >200 | 330 | 180 | 30 |
| Tg (ºC) | _110 | _100 | _10 | 80 | 90 | 95 | _110 | 105 | _70 |
Polymer Categories
Thermoplastics
Most of the polymers described in this chapter are classified as thermoplastics. Plastics that soften when heated and become firm again when cooled. This is the more popular type of plastic because the heating and cooling may be repeated and the thermoplastic may be reformed. These polymers tend to have high Tg so they are hard solids at room temperature, however, above Tg they they become malleable and may be shaped, pressed into molds, spun, or cast from melts.
Polyethylene (poly(ethylene terephthalate) or PET is the most common thermoplastic . In 2017, PET made up 34% of the total plastics market with over 100 million tones of polyethylene resins being produced. PET is partially crystalline and is used to create clear plastic bottles such as 2-liter beverage bottles, milk jugs, detergent bottles, and water bottles.
Polystyrene is also a common thermoplastic. The polymer is making it a solid but rather rather brittle at room temperature. Polystyrene is used to make hard clear plastic cups, foam cups, eating utensils, deli food containers, toy model kits, some packing popcorn.
Plasticizers
Plasticizers or dispersants are additives that increase the plasticity or decrease the viscosity of a material. These substances are compounded into certain types of plastics to render them more flexible by lowering the glass transition temperature. They accomplish this by taking up space between the polymer chains and acting as lubricants to enable the chains to more readily slip over each other. Many (but not all) are small enough to be diffusible and a potential source of health problems. Substantial concerns have been expressed over the safety of some plasticizers, especially because some low molecular weight ortho-phthalates have been classified as potential endocrine disruptors with some developmental toxicity reported.
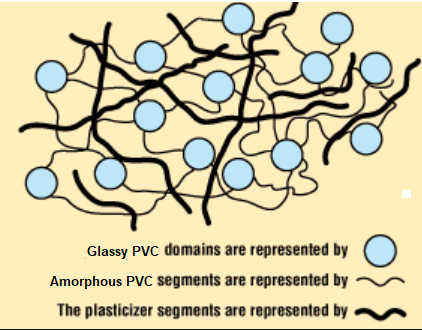
Polyvinyl chloride (PVC) polymers are one of the most widely-plasticized types. PVC is usually is not very crystalline and is relatively brittle and glassy. The properties of polyvinyl chloride can be improved by blending it with substances of low volatility which tend to break down its glasslike structure. Common plasticizers used with PVC are tris-(2-methylphenyl) phosphate (tricresyl phosphate) and dibutyl benzene-1,2-dicarboxylate (dibutyl phthalate). Plasticized polyvinyl chloride is reasonably flexible and is widely used for flexible vinyl materials such as garden hoses, waterbeds, cheap shower curtains, raincoats and upholstery. The pungent oder associated with these products are a testament to the ability of plasticizers to migrate into the environment.
Fibers
Fibers are drawn into thin threads by forcing the melted or dissolved polymer through a spinneret to generate filaments that can be woven into fabrics. Part of this process, called cold-drawing, the polymer material is subjected to strong stress in one direction causing the material to elongate and the crystallites to be drawn together and oriented along the direction of the applied stress. Having the crystallites in a polymer oriented with respect to one another gives the polymer a much higher tensile strength than an unoriented polymer. Polymers such as nylon, which has strong intermolecular forces, has the crystallinity required to be drawn in oriented fibers.
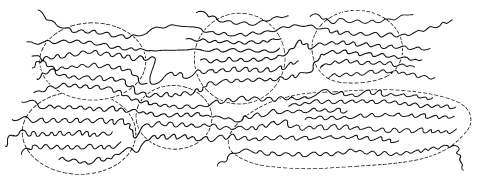
Schematic representation of an oriented crystalline polymer produced by drawing the polymer in the horizontal direction. The crystalline regions are enclosed with dashed lines.
Elastomers
Elastomers usually are amorphous polymers which have the ability to be stretched. The key to this elastic behavior is polymer chains with weak forces between the chains and a sufficiently irregular structure to be unstable in the crystalline state. A useful elastomer needs to have some kind of cross-linking. The important difference between an elastomer and a crystalline polymer is the size of the amorphous regions. When tension is applied and the material elongates, the chains in the amorphous regions straighten out and become more nearly parallel. The forces between the chains are too weak to maintain the crystalline state in the absence of tension. Thus when tension is released, contraction occurs and the original, amorphous polymer is produced. The entropy of the chains is more favorable in the relaxed state than in the stretched state.
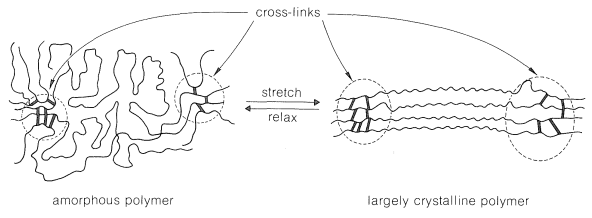
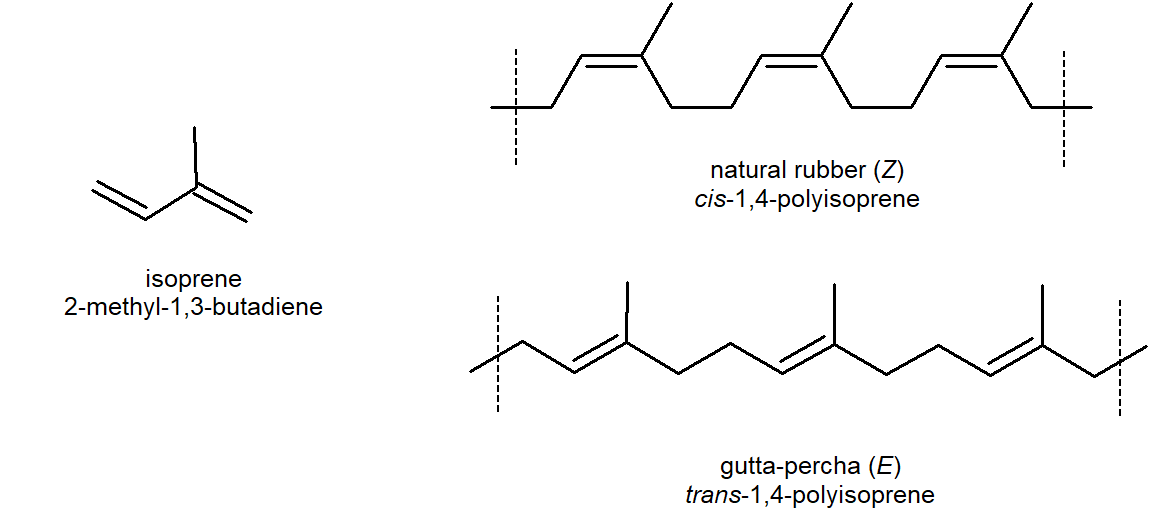
A good elastomer should not undergo plastic flow in either the stretched or relaxed state, and when stretched should have a "memory" of its relaxed state. These conditions are best achieved with natural rubber (cis-poly-2-methyl-1,3-butadiene, cis-polyisoprene) by curing (vulcanizing) with sulfur. Natural rubber (Section 14-6) is tacky and undergoes plastic flow rather readily, but when it is heated with elemental sulfur, sulfur cross-links are introduced between the chains. These cross-links reduce plastic flow and provide a reference framework for the stretched polymer to return to when it is allowed to relax. Also the double bonds in rubber all have a Z-configuration, which causes this macromolecule to adopt a kinked or coiled conformation.
However, the gutta-percha (structure above) E-isomer of rubber adopts a uniform zig-zag conformation, which produces greater crystallinity making it not an elastomer.
Thermosets
Thermosets are plastics that soften when heated and can be molded, but harden permanently. In a thermoset, crosslinks connect the different chains in the material, forming bridges that span from chain to chain to chain, essentially uniting the material into one big molecule. If it is one big molecule, the chains can never move completely independently of each other, and the material cannot form a new shape.
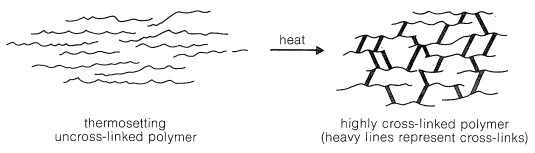
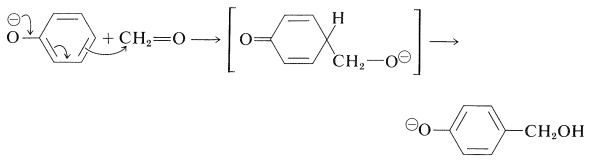
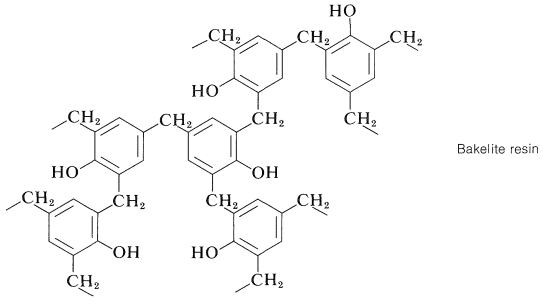
One of the oldest known thermosetting synthetic polymers, Bakelite, is made by condensation of phenol with formaldehyde. During the thermoset process water is lost and many crosslinks are formed. produce (4-hydroxyphenyl)methanol. Bakelite was patented on December 7, 1909 and was revolutionary for its electrical nonconductivity and heat-resistant properties used in electrical insulators, radio and telephone casings and such diverse products as kitchenware, jewelry, pipe stems, and children's toys.
Propose a mechanism for the base-catalyzed polymerization of phenol and formaldehyde to form Bakelite.
- Answer
-
One of the oldest known thermosetting synthetic polymers is made by condensation of phenols with aldehydes using basic catalysts. The resins that are formed are known as Bakelites. The initial stage is the base-induced reaction of benzenol and methanal to give a (4-hydroxyphenyl)methanol, and this reaction closely resembles an aldol addition and can take place at either the 2- or the 4-position of the benzene ring:
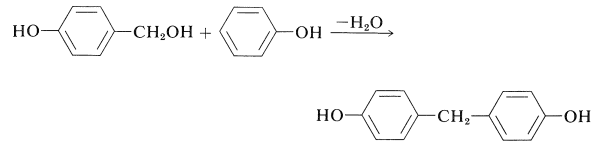
The next step in the condensation is formation of a bis(hydroxyphenyl)methane derivative, which for convenience is here taken to be the 4,4'-isomer:
This reaction is probably a Michael type of addition to a base-induced dehydration product of the (4-hydroxyphenyl)methanol:
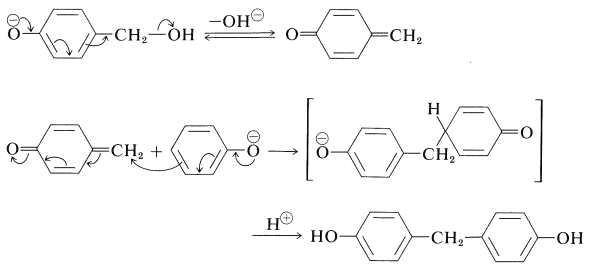
Continuation of these reactions at the 2-, 4-, and 6-positions of the benzenol leads to the cross-linked three-dimensional Bakelite resin:
Would you expect the catalytic hydrogenation of gutta-percha to produce a product that is syndiotactic, atactic, or isotactic?
- Answer
-
Atactic. The methyl groups in gutta-percha lack stereochemistry because they are attached to double bond. Catalytic hydrogenation does not provide stereochemical control so H2 could attack from either side of the double bond to produce a chiral center involving the methyl group which is R or S.

