30.3: Copolymers
- Page ID
- 364613
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
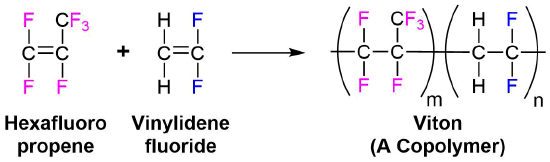
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Homopolymers are made with a single monomer and are made up of identical repeating units. Copolymers is made when two or more different monomers are polymerized together to create a polymer with variable repeating units. For example the monomers hexafluoropropene and vinylidene fluoride can be polymerized together to create the copolymer vitron which is used to create durable gaskets.
The synthesis of macromolecules composed of more than one repeating unit has been explored as a means of controlling the properties of the resulting material. In this respect, it is useful to distinguish several ways in which different monomeric units might be incorporated in a polymer. The following examples refer to a two component system, in which one monomer is designated A and the other B.
Statistical Copolymers
Also called random copolymers. Here the monomeric units are distributed randomly, and sometimes unevenly, in the polymer chain:
~ABBAAABAABBBABAABA~
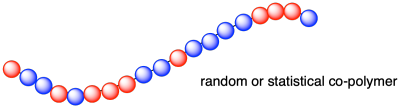
Most direct copolymerizations of equimolar mixtures of different monomers give statistical copolymers. If you take a mixture of alkenes that are capable of forming polymers and you polymerize them together, you may well get them randomly enchained into a growing polymer. For example, polymerizing propene and vinyl chloride together creates a polymer with random monmer unit.

Alternating Copolymers
Here the monomeric units are distributed in a regular alternating fashion, with nearly equimolar amounts of each in the chain:
~ABABABABABABABAB~
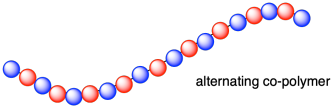
Formation of alternating copolymers is favored when the monomers have different polar substituents (e.g. one electron withdrawing and the other electron donating), and both have similar reactivities toward radicals. For example, styrene and acrylonitrile copolymerize in a largely alternating fashion.
In some of the examples alternating copolymers, the chain is actually composed of two different monomers. This is the case in polyamides such as nylon-6,6, which is a chain is composed of difunctional amines alternating with difunctional carboxyloids (such as carboxylic acids or acid chlorides). Because of their complementary reactivity, the monomers have to alternate: an amine and then a carboxyloid, to form an amide, and so on. We can think of these polymers as "alternating co-polymers" because the two different monomers alternate with each other along the chain.

Block Copolymers
Instead of a mixed distribution of monomeric units, a long sequence or block of one monomer is joined to a block of the second monomer:
~AAAAA-BBBBBBB~AAAAAAA~BBB~
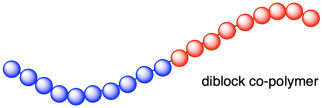
Several different techniques for preparing block copolymers have been developed, with a common example being anionic polymerization. In the anionic polymerization of styrene, a reactive site remains at the end of the chain until it is quenched. The unquenched polymer has been termed a living polymer because the polymerization can continue as long as monomer is present. If different suitable monomer, methyl methacrylate, is added the chain will continue grow by adding methyl methacrylate units and a block polymer will form. This is illustrated for in the following diagram.

Graft Copolymers
As the name suggests, side chains of a given monomer are "grafted" to the main chain of a different monomer:
~AAAAAAA(BBBBBBB~)AAAAAAA(BBBB~)AAA~.
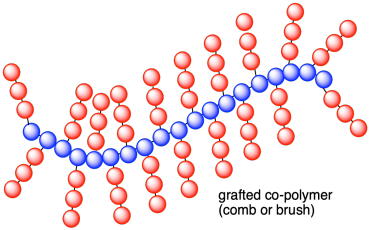
Graft polymers can be made in great profusion by attaching chains of one kind of polymer to the middle of another. A particularly simple but uncontrollable way of doing this is to knock groups off a polymer chain with x-ray or alpha radiation in the presence of a monomer. The polymer radicals so produced then can grow side chains made of the new monomer. A more elegant procedure is to use a photochemical reaction to dissociate groups from the polymer chains and form radicals capable of polymerization with an added monomer.
| Monomer A | Monomer B | Copolymer | Uses |
|---|---|---|---|
| H2C=CHCl | H2C=CCl2 | Saran | films & fibers |
| H2C=CHC6H5 | H2C=C-CH=CH2 |
SBR |
tires |
| H2C=CHCN | H2C=C-CH=CH2 | Nitrile Rubber |
adhesives |
| H2C=C(CH3)2 | H2C=C-CH=CH2 | Butyl Rubber | inner tubes |
| F2C=CF(CF3) | H2C=CHF | Viton | gaskets |
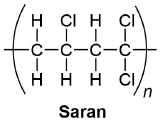
Draw the structure of an alternating segment of Saran, a copolymer of vinyl chloride (chloroethene) and vinylidene chloride (1,1-dichloroethene).
- Answer
-