29.6: Stereochemistry of Cycloadditions
- Page ID
- 364601
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Frontier orbital theory can be used to predict if a given cycloaddition will occur with suprafacial or with antarafacial geometry. In a standard Diels-Alder reaction, bonding interactions are created when the electron containing HOMO of the diene donates electrons to the electron vacant LUMO of the other the dienophile. The dienophile has one pi bond, so it will use the pi MOs for a 2 atom system. The dienophile has 2 pi electrons which makes psi 2* its LUMO. The diene has two pi bonds, so it will use the pi MOs for a 4 atom system. The diene has has 4 pi electrons makes psi 2 its HOMO.
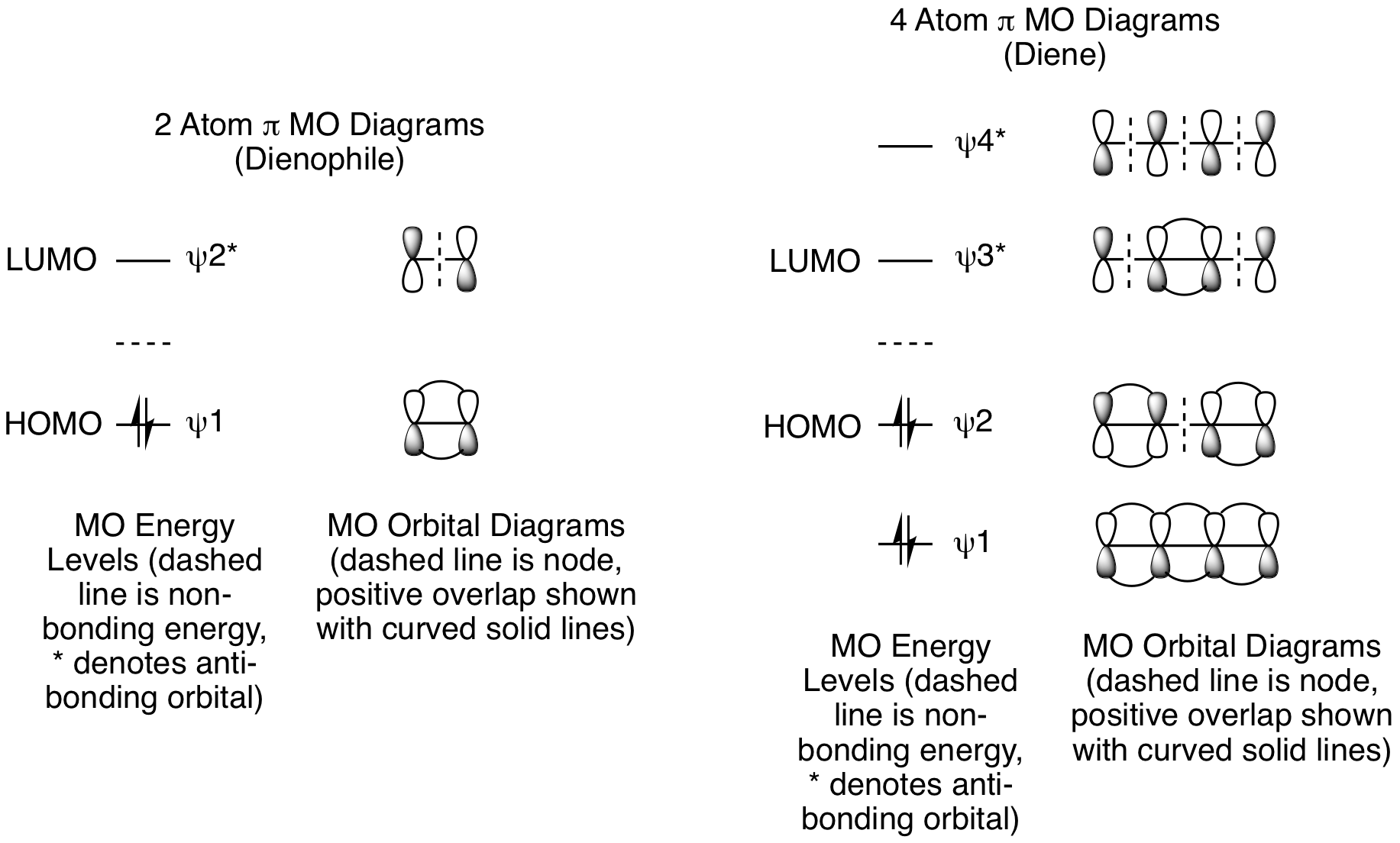
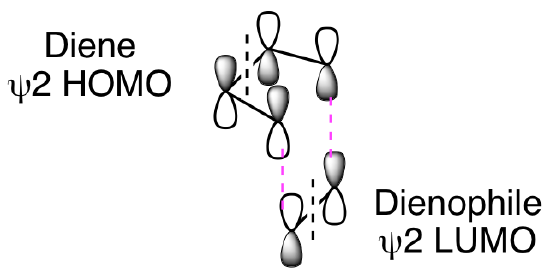
These MO diagrams show that the ground-state frontier orbitals of both reactants have terminal lobes with matching signs. The symmetry of these orbitals are such that bond formation will easily occur under thermal conditions with suprafacial geometry as shown below. (Note: The dashed black lines in the figure below represent nodes in the pi molecular orbitals of the diene and dienophile.) The two new sigma bonds, shown as dashed magenta lines below, are formed from constructive overlap of the terminal dienophile orbitals with the terminal orbitals of the diene.
Photochemical [2+2] cycloadditions are excellent reactions for the synthesis of strained products containing 4-membered rings. One of the reaction partners must be conjugated so that it can absorb light and become an excited state molecule. These reactions produce strained 4-membered rings but are not reversible because the products lack conjugation and, thus, can't absorb light to facilitate a cycloreversion.
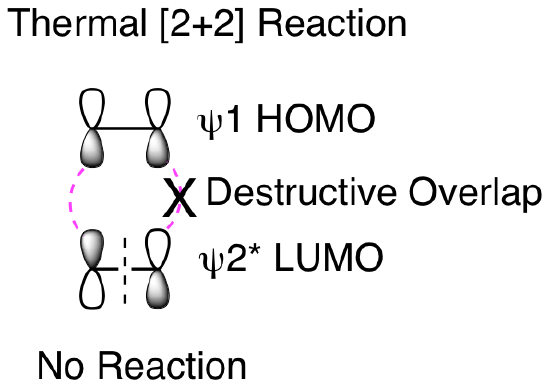
So, why won't this reaction happen thermally? How does our molecular orbital analysis help us understand the importance of this being a photochemical reaction? First, let's look at the orbital analysis if we tried to do a thermal [2+2] reaction. As shown below, we cannot get suprafacial overlap for both of the 2 pi reactants when trying to combine psi 1 HOMO with psi 2* LUMO. This means that it is not favorable to convert the two reactant pi bonds into two new product pi bonds.
What happens when we shine light on the reaction? Light creates an excited state molecule by promoting an electron in the HOMO to the LUMO, as shown below. This means the excited state HOMO is the ground state LUMO. We need to understand a few key points about photoreactions before doing our molecular orbital analysis. Excited state molecules are very short lived, relaxing back to the ground state very quickly. Therefore, it is practically impossible for two excited state molecules to find each other in a reaction. Instead, reactions occur between one excited state molecule and one ground state molecule. When only one molecule is conjugated, that is the molecule that will form the excited state. If both reactants are conjugated, either can form the excited state. The orbital analysis is shown below. First, we see the orbital picture when a ground state molecule absorbs light to form an excited state. Second, when we analyze the reaction, it is now psi 2* HOMO of the excited state molecule reacting with psi 2* LUMO of the ground state molecule. This gives suprafacial constructive overlap for both orbitals and the 2 reactant pi bonds can be converted into two product sigma bonds.
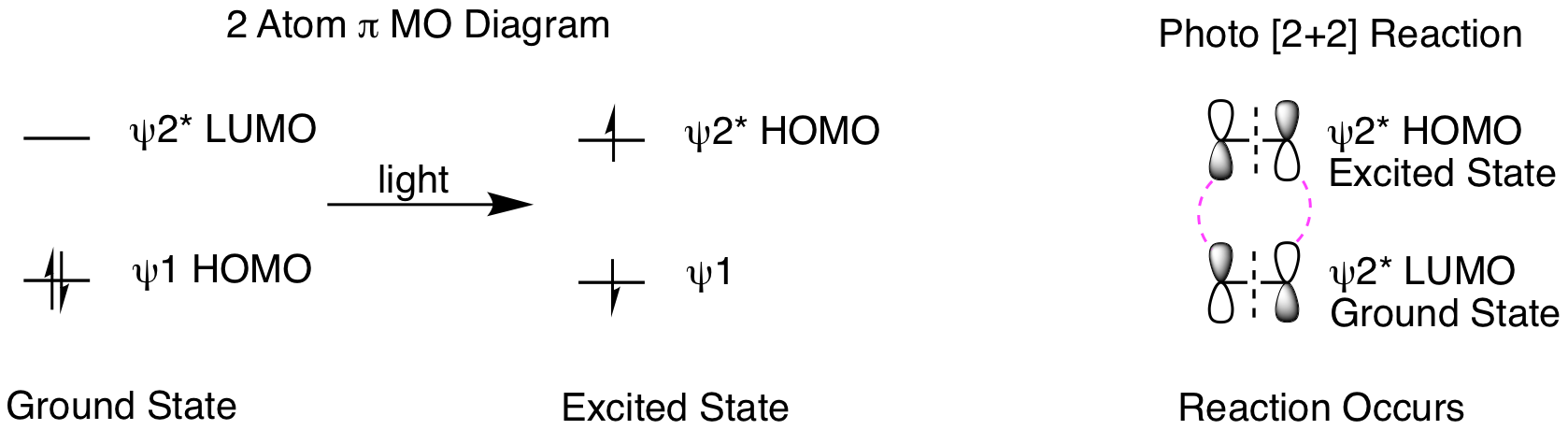
Cycloaddition reactions can be categorized based on the total number electrons involved in the rearrangement. The [4+2] Diels-Alder reaction involves 6 electrons and takes place using a suprafacial pathway under thermal conditions. The thermal [2+2] cycloaddtion of two alkenes involves 4 electrons and must take place by antarafacial pathway. However, the pathway is reversed for photochemical [2+2] cycloaddtions which take place by a supraficial pathway. These ideas can be generalized using the rules for cycloadditions below.
| Number of Electrons | Thermal | Photochemical |
|---|---|---|
| 4n + 2 | Suprafacial (Allowed) | Antarafacial (Forbidden) |
| 4n | Antarafacial (Forbidden) | Suprafacial (Allowed) |
For the following reactions determine what type of cycloaddition is occurring, is the reaction supra or antarafacial, and would the reaction require thermal or photochemical conditions.
a)
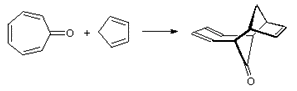
b)
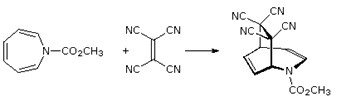
c)
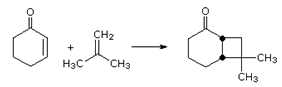
- Answer
-
a) [6+4], suprafacial, thermal
b) [4+2], suprafacial, thermal
c) [2+2], antarafacial, photochemical


