7.2: Industrial Preparation and Use of Alkenes
- Page ID
- 31451
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- discuss the industrial importance of ethylene (ethene) and propylene (propene).
- describe, briefly, the industrial process known as thermal cracking.
Among the most important and most abundant organic chemicals produced worldwide are the two simple alkenes, ethylene and propylene. They are used as the starting materials to synthesize numerous valuable compounds.
Produced from ethylene (ethene)
| Chemical | Uses |
|---|---|
| ethanol | solvent; constituent of cleaning preparations; in synthesis of esters |
| acetaldehyde | slug killer, in the form of methaldehyde (CH3CHO)4 |
| acetic acid | manufacture of vinyl acetate polymers, ethyl acetate solvent and cellulose acetate polymers |
| ethylene oxide | “cellosolves” (industrial solvents) |
| ethylene glycol | anti-freeze; production of DacronOR |
| ethylene dichloride | solvent; production of vinyl chloride |
| vinyl chloride | manufacture of poly (vinyl chloride)—PVC |
| vinyl acetate | manufacture of poly (vinyl acetate) used in paint emulsions, plywood adhesives and textiles |
| polyethylene | “plastic” bags; toys; packaging |
Produced from propylene (propene)
| Chemical | Uses |
|---|---|
| isopropyl alcohol | rubbing alcohol; cosmetics; synthesis of acetone |
| propylene oxide | manufacture of polyurethanes; polyesters |
| cumene | industrial preparation of phenol and acetone |
| polypropylene | molded articles (e.g., kitchenware); fibres for indoor-outdoor carpeting |
Industrial Preparation of Ethylene and Propylene
Ethene (CH2CH2) and propene (CH3CHCH2), are most often called by their common names—ethylene and propylene. Ethylene is a major commercial chemical. The US chemical industry produces about 25 billion kilograms of ethylene annually, more than any other synthetic organic chemical. More than half of this ethylene goes into the manufacture of polyethylene, one of the most familiar plastics. Propylene is also an important industrial chemical. It is converted to plastics, isopropyl alcohol, and a variety of other products. Both ethylene and propylene are the feedstock for the industrial synthesis of a wide variety of small organic molecules.
Ethylene, propylene, and butylene (CH3CH2CHCH2) are typically industrially synthesized through the steam cracking of light alkanes (C < 8) obtained from fractional distillation of crude oil. Cracking is the name given to a number of petroleum refining processes which break up large hydrocarbon molecules into smaller fragment. Steam cracking is achieved without a catalyst by using high temperatures (~900 oC) and produces a mixtures of products containing high proportions of hydrocarbons with double bonds. There is not any single unique reaction happening during steam cracking. The hydrocarbon molecules are broken up in a fairly random way but the process can be generically represented by the reaction below.
The mechanism of the steam cracking is complex and believed to involve the formation of free radicals. The high temperatures of steam cracking is enough to cause the homolytic cleavage of C-C and C-H bonds in the starting material. The cleavage of C-C bonds inherently creates smaller hydrocarbons as represented below.
Industrial Preparations of Alcohols from Ethylene and Propylene
Ethanol is manufactured by reacting ethylene with steam. The catalyst used is solid silicon dioxide coated with phosphoric acid. The reaction is reversible.
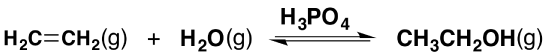

Only 5% of the ethylene is converted into ethanol at each pass through the reactor. By removing the ethanol from the equilibrium mixture and recycling the ethylene, it is possible to achieve an overall 95% conversion.
Isopropyl alcohol is synthesized on an industrial scale using a similar reaction with propylene.
Ethylene is also used in the industrial synthesis of ethylene glycol. Ethylene glycol is a major industrial compound with a wide range of applications. The traditional method to synthesize ethylene glycol is a two step process in which ethylene is directly oxidized to ethylene oxide. Next the three-membered ring of ethylene oxide is opened using water at high temperatures and pressures to form ethylene glycol.
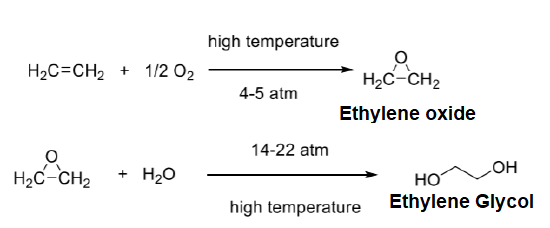
Industrial Preparations of Plastics from Ethylene and Propylene
One of the primary uses of industrial synthesized ethylene is the production of the polymer polyethylene (PE). Polyethylene is the most common plastic. As of 2017, over 100 million tons of polyethylene resins are produced annually, accounting for 34% of the total plastics market. Its primary use is in packaging (plastic bags, plastic films, geomembranes, containers including bottles, etc.). Many kinds of polyethylene are known, with most having the chemical formula (C2H4)n .
It is the simplest polymer, consisting of random-length (but generally very long) chains made up of two-carbon units. During the polymerization process many ethylene molecules are linked together to form the carbon backbone of polyethylene. In the polyethylene structures are represented below. The squiggly lines at the ends of the long structure indicate that the same pattern extends indefinitely. The more compact notation on the right shows the minimal repeating unit enclosed in brackets; this means the same thing and is the preferred way of depicting polymer structures.


