3.5: Properties of Alkanes
- Page ID
- 31402
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- arrange a number of given straight-chain alkanes in order of increasing or decreasing boiling point or melting point.
- arrange a series of isomeric alkanes in order of increasing or decreasing boiling point.
- explain the difference in boiling points between a given number of alkanes.
Make certain that you can define, and use in context, the key term below.
- van der Waals force (also known as London Dispersion force)
Alkanes are not very reactive and have little biological activity; all alkanes are colorless and odorless non-polar compounds. The relative weak London dispersion forces of alkanes result in gaseous substances for short carbon chains, volatile liquids with densities around 0.7 g/mL for moderate carbon chains, and solids for long carbon chains. For molecules with the same functional groups, there is a direct relationship between the size and shape of molecules and the strength of the intermolecular forces (IMFs) causing the differences in the physical states.
Boiling Points
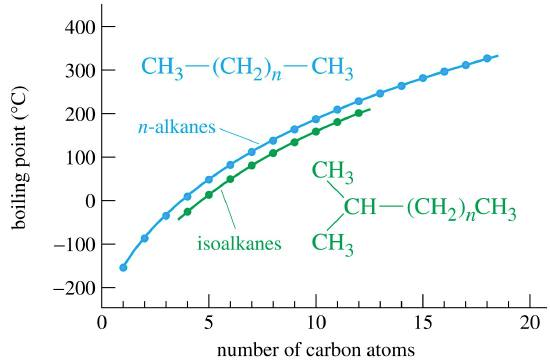
Table \(\PageIndex{1}\) describes some of the properties of some straight-chain alkanes. There is not a significant electronegativity difference between carbon and hydrogen, thus, there is not any significant bond polarity. The molecules themselves also have very little polarity. A totally symmetrical molecule like methane is completely non-polar, meaning that the only attractions between one molecule and its neighbors will be Van der Waals dispersion forces. These forces will be very small for a molecule like methane but will increase as the molecules get bigger. Therefore, the boiling points of the alkanes increase with molecular size.
For isomers, the more branched the chain, the lower the boiling point tends to be. Van der Waals dispersion forces are smaller for shorter molecules and only operate over very short distances between one molecule and its neighbors. It is more difficult for short, fat molecules (with lots of branching) to lie as close together as long, thin molecules.
The boiling points shown are for the "straight chain" isomers of which there is more than one. The first four alkanes are gases at room temperature, and solids do not begin to appear until about \(C_{17}H_{36}\), but this is imprecise because different isomers typically have different melting and boiling points.3.2.1
| Molecular Name | Formula | Melting Point (°C) | Boiling Point (°C) | Density (20°C)* | Physical State (at 20°C) |
|---|---|---|---|---|---|
| methane | CH4 | –182 | –164 | 0.668 g/L | gas |
| ethane | C2H6 | –183 | –89 | 1.265 g/L | gas |
| propane | C3H8 | –190 | –42 | 1.867 g/L | gas |
| butane | C4H10 | –138 | –1 | 2.493 g/L | gas |
| pentane | C5H12 | –130 | 36 | 0.626 g/mL | liquid |
| hexane | C6H14 | –95 | 69 | 0.659 g/mL | liquid |
| octane | C8H18 | –57 | 125 | 0.703 g/mL | liquid |
| decane | C10H22 | –30 | 174 | 0.730 g mL | liquid |
| *Note the change in units going from gases (grams per liter) to liquids (grams per milliliter). Gas densities are at 1 atm pressure. | |||||
The boiling points for the "straight chain" isomers and isoalkanes isomers are shown to demonstrate that branching decreases the surfaces area, weakens the IMFs, and lowers the boiling point.
For example, the boiling points of the three isomers of \(C_5H_{12}\) are:
- pentane: 309.2 K
- 2-methylbutane: 301.0 K
- 2,2-dimethylpropane: 282.6 K
The slightly higher boiling points for the cycloalkanes are presumably because the molecules can get closer together because the ring structure makes them better able!
For each of the following pairs of compounds, select the substance which you expect to have the higher boiling point:
- octane and nonane.
- octane and 2,2,3,3-tetramethylbutane.
Solution
- nonane, since it has more atoms it will have greater IMF
- octane, since it is not branched, the molecules can pack closer together increasing IMF
Solubility
Alkanes are virtually insoluble in water, but dissolve in organic solvents. However, liquid alkanes are good solvents for many other non-ionic organic compounds.
Solubility in Water
When a molecular substance dissolves in water, the following must occur:
- break the intermolecular forces within the substance. In the case of the alkanes, these are the Van der Waals dispersion forces.
- break the intermolecular forces in the water so that the substance can fit between the water molecules. In water, the primary intermolecular attractions are hydrogen bonds.
Breaking either of these attractions requires energy, although the amount of energy to break the Van der Waals dispersion forces in something like methane is relatively negligible; this is not true of the hydrogen bonds in water.
As something of a simplification, a substance will dissolve if there is enough energy released when new bonds are made between the substance and the water to compensate for what is used in breaking the original attractions. The only new attractions between the alkane and the water molecules are Van der Waals forces. These forces do not release a sufficient amount of energy to compensate for the energy required to break the hydrogen bonds in water. The alkane does not dissolve.
This is a simplification because entropic effects are important when things dissolve.
Solubility in organic solvents
In most organic solvents, the primary forces of attraction between the solvent molecules are Van der Waals. Therefore, when an alkane dissolves in an organic solvent, the Van der Waals forces are broken and are replaced by new Van der Waals forces. The two processes more or less cancel each other out energetically; thus, there is no barrier to solubility.
For each of the following pairs of compounds, select the substance you expect to have the higher boiling point.
- octane and nonane.
- octane and 2,2,3,3‑tetramethylbutane.
- Answer
-
Nonane will have a higher boiling point than octane, because it has a longer carbon chain than octane. Octane will have a higher boiling point than 2,2,3,3‑tetramethylbutane, because it branches less than 2,2,3,3‑tetramethylbutane, and therefore has a larger “surface area” and more van der Waals forces.
Note: The actual boiling points are:
- nonane, 150.8°C
- octane, 125.7°C
- 2,2,3,3‑tetramethylbutane, 106.5°C
Reactions of Alkanes
Alkanes undergo very few reactions. There are two important reactions that are still possible, combustion and halogenation. The halogenation reaction is very important in organic chemistry because it opens a gateway to further chemical reactions.
Combustion
Complete combustion (given sufficient oxygen) of any hydrocarbon produces carbon dioxide, water, and a significant amount of heat. Due to the exothermic nature of these combustion reactions, alkanes are commonly used as a fuel source (for example: propane for outdoor grills, butane for lighters). The hydrocarbons become harder to ignite as the molecules get bigger. This is because the larger molecules don't vaporize as easily. If the liquid is not very volatile, only those molecules on the surface can react with the oxygen. Larger molecules have greater Van der Waals attractions which makes it more difficult for them to break away from their neighbors and become a gas. An example combustion reaction is shown for propane:
\[ \ce{ C_3H_8 + O_2 -> 3CO_2 + 4H_2O + 2044 kJ/mol} \nonumber \]
Halogenation
Halogenation is the replacement of one or more hydrogen atoms in an organic compound by a halogen (fluorine, chlorine, bromine or iodine). Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in which a C-H bond is broken and a new C-X bond is formed.
Since only two covalent bonds are broken (C-H & Cl-Cl) and two covalent bonds are formed (C-Cl & H-Cl), this reaction seems to be an ideal case for mechanistic investigation and speculation. However, one complication is that all the hydrogen atoms of an alkane may undergo substitution, resulting in a mixture of products, as shown in the following unbalanced equation. The relative amounts of the various products depend on the proportion of the two reactants used. In the case of methane, a large excess of the hydrocarbon favors formation of methyl chloride as the chief product; whereas, an excess of chlorine favors formation of chloroform and carbon tetrachloride.
\[\ce{CH_4 + Cl_2 \rightarrow CH_3Cl + CH_2Cl + CHCl_3 + CCl_4 + HCl} \nonumber \]
An understanding of the physical properties of alkanes is important since petroleum and natural gas and the many products derived from them—gasoline, bottled gas, solvents, plastics, and more—are composed primarily of alkanes. This understanding is also vital because it is the basis for describing the properties of other organic and biological compound families. For example, large portions of the structures of lipids consist of nonpolar alkyl groups. Lipids include the dietary fats and fat like compounds called phospholipids and sphingolipids that serve as structural components of living tissues. These compounds have both polar and nonpolar groups, enabling them to bridge the gap between water-soluble and water-insoluble phases. This characteristic is essential for the selective permeability of cell membranes.
Tripalmitin (a), a typical fat molecule, has long hydrocarbon chains typical of most lipids. Compare these chains to hexadecane (b), an alkane with 16 carbon atoms.

