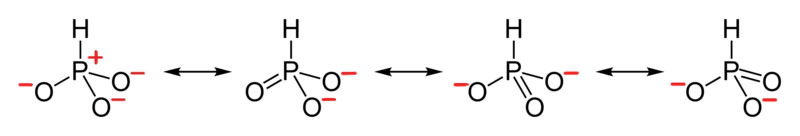
2.5: Drawing Resonance Forms
- Page ID
- 31388
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- draw the resonance structures of molecules or ions that exhibit delocalization.
- determine the relative stability of resonance structures using a set of rules.
- use the concept of resonance to explain structural features of molecules and ions.
- resonance structure
- resonance hybrid
Curved Arrows Communicate Electron Flow (movement)
Organic chemistry has developed a system to show how electrons move between resonance structures. This system will also be used to help describe how electrons from in reactions. Curved double barbed arrows indicates the flow of two electrons. The base of the curved arrow is placed at the source of the electrons that are moving. The head of the arrow is placed at the destination of the electrons.
It is also important to consciously use the correct type of arrow. There are four primary types of arrows used by chemists to communicate one of the following: completion reaction, equilibrium reaction, electron movement, and resonance forms. The three other types of arrows are shown below to build discernment between them. Note, the electron movement arrows are the only ones that are curved.
Reaction Arrows
Resonance Arrows
Using Curved Arrows to Show Electron Movement
Because the double barbed arrow represents the movement of two electrons, they usually involve lone pair electrons or pi bonds. There are only three types of electron "motion" in resonance. They are:
- A lone pair forms a pi bond to an adjacent atom
- A pi bond forms a new pi bond to an adjacent atom
- A pi bond forms a lone pair on adjacent atom
Let's look at the resonance within acrylic acid to demonstrate these three types of resonance.
The curved arrow in structure A represents the type 3 resonance "motion" - the pi bond between the carbon and oxygen breaks to form another lone pair on the oxygen. The curved arrow in structure B represents type 2 resonance "motion" - the pi bond breaks to form a new pi bond to the carbocation carbon. In structure C, there are two curved arrows. The curved arrow from the oxygen lone pair is type 1 resonance motion - the lone pairs forms a new pi bond between the oxygen and carbon. The other arrow in structure C moves the pi bond to the end of the chain and represents resonance type 2. By combining these three basic types of electron movement we can describe virtually any type of resonance.
Example:
Below are a few more examples of ‘legal’ resonance expressions. Confirm for yourself that the octet rule is not exceeded for any atoms, that formal charges are correct, and identify which type of electron movement is being represented by each arrow.
Draw the resonance contributors that correspond to the curved, two-electron movement arrows in the resonance expressions below. Then identify the type of resonance motion in each structure below.
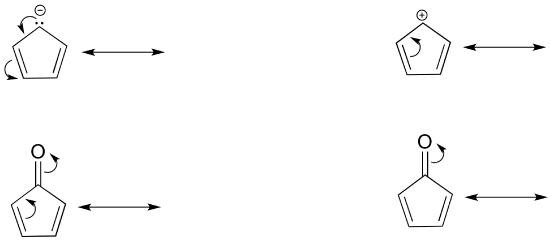
- Answer
-
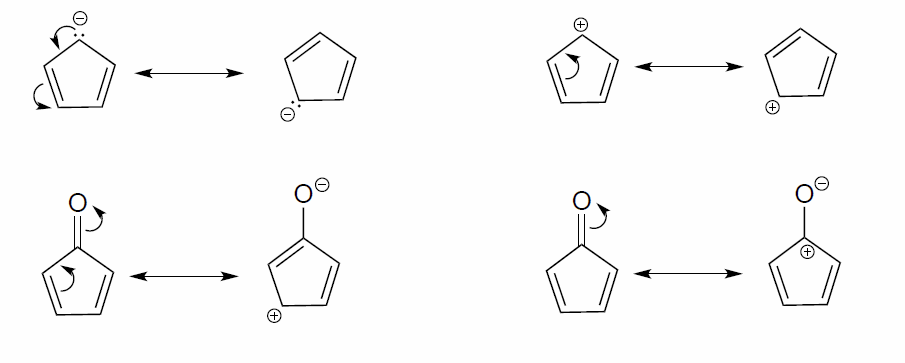
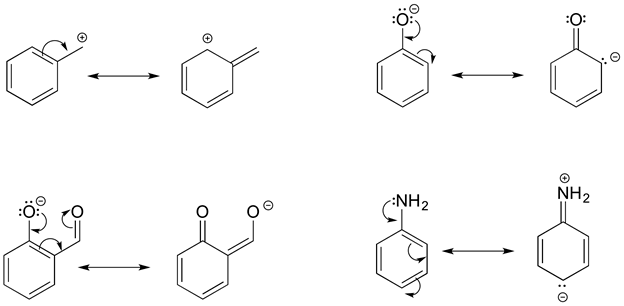
In each resonance expression, identify the type of resonance motion. Then draw curved arrows on the left-side contributor that shows how we get to the right-side contributor.
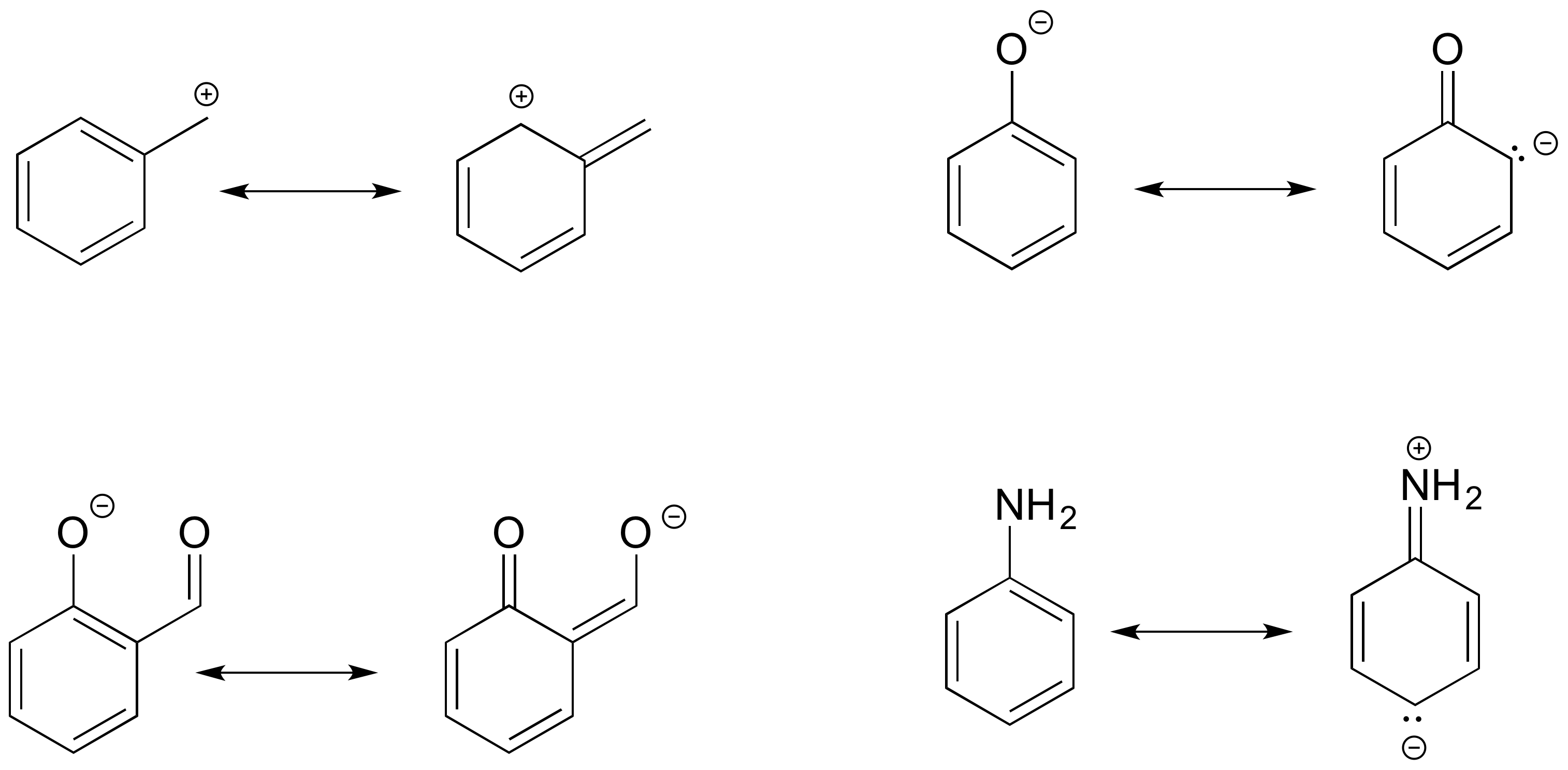
- Answer
-
Recognizing Common Patterns of Resonance
If you examine a large number of resonance examples, you will begin to notice that they nearly always match common patterns, of which there are only three. It is important to be able to identify atoms that participate in resonance. In complex resonance cases, multiple types of resonance may occur simultaneously.
Type I - Neutral Species
The electrons of a pi bond move to become a set of lone pair electrons on a electronegative atom. The resonance structure made has a carbon with a violated octet which make it a minor contributor. This type of resonance is commonly used to the polarity in certain double bonds.
Neutral Species
Note: Y is an electronegative atom, usually N, O, or S.
Type II - Charged Species
Type II resonance is only seen with a + charge, and usually involves a positive charge on oxygen or nitrogen being shared onto a carbon; the carbocation form has only six valence electrons on the carbon, so it is a less stable form than the major form (which has complete octets).
Charged Species
Note: Y is an electronegative atom, usually N, O, S, sometimes halogen
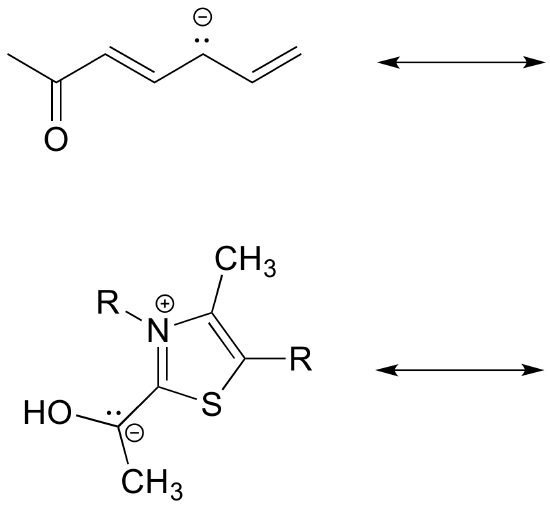
Type III - Allylic Resonance
Type III resonance is very common and important because is serves to stabilize positive charges, negative charges, or lone pairs. It is sometimes referred to as “allylic” resonance, especially in cases with all carbon. This type of resonance can be identified by a three-atom group of atoms each with sp2 hybridization and a p orbital.
* represents a charge or an unpaired electron
Atoms with lone pair electrons next to a pi bond can be sp2 hybridized and have the lone pair of electrons in a p orbital despite the fact that they are surrounded by four electron groups. The lone pair electrons contained in the p orbital cause the ion to be stabilized due to resonance.
Similarly, carbocations are sp2-hybridized, with an empty 2p orbital oriented perpendicular to the plane formed by three sigma bonds. If a carbocation is adjacent to a double bond, then three 2p orbitals can overlap and share the two pi electrons - another kind of conjugated pi system in which the positive charge is shared over two carbons.
charge is delocalized over two carbons
Each of the 'illegal' resonance expressions below contains one or more mistakes. Explain what is incorrect in each.
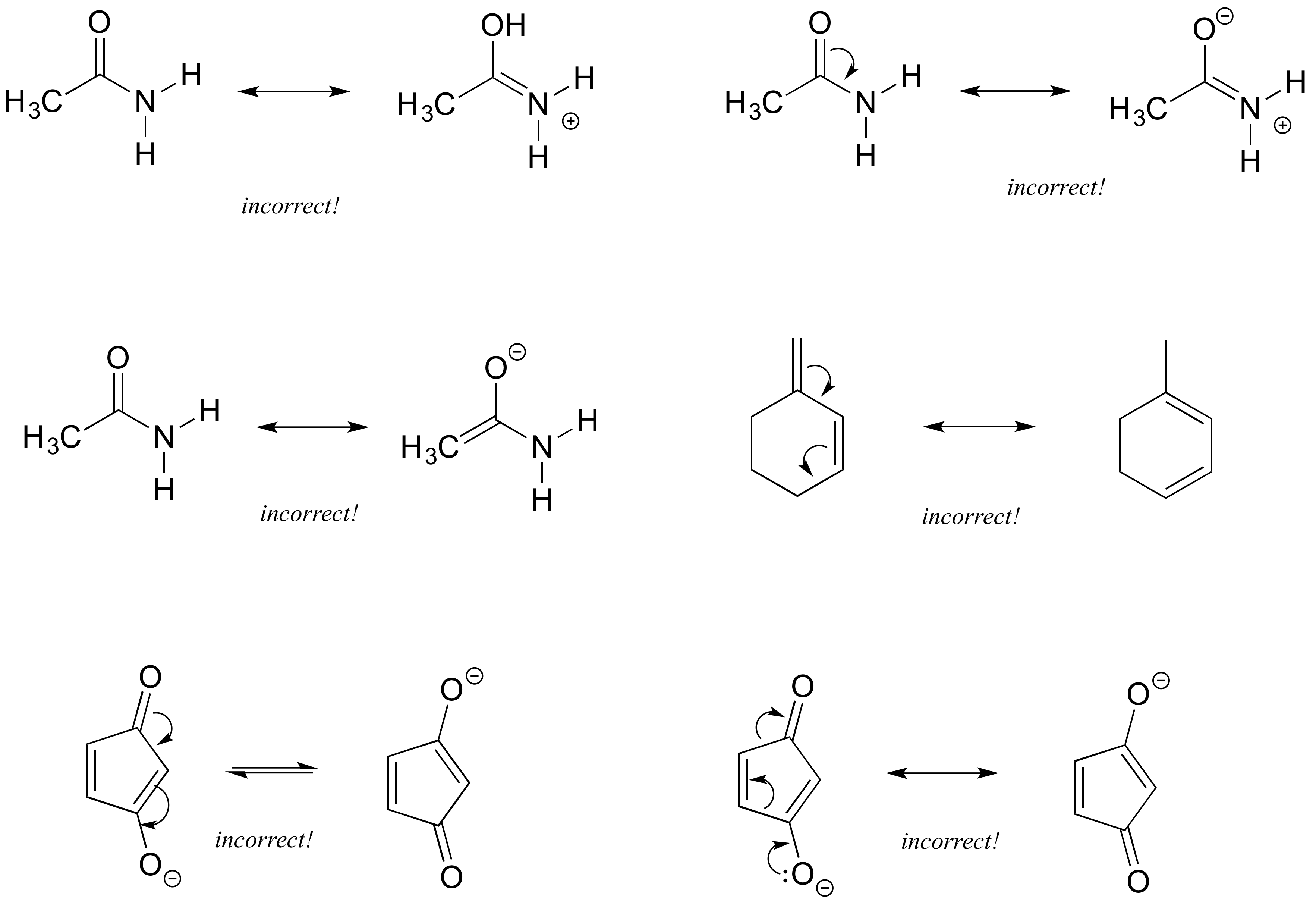
- Answer
-
The first pair are not resonance structures since there is an additional hydrogen on the second structure oxygen. The second pair pushed electrons toward nitrogen which already has a lone pair and would exceed its octet. The third pair includes a structure with 5 bonds to carbon. The fourth pair requires moving carbon-hydrogen bonds, therefore is not resonance. The fifth pair show electrons moving toward the negatively charged oxygen which would exceed an octet. The fifth pair shows a sigma bond breaking on the ring, rather than pi bond.
a) Draw three additional resonance contributors for the carbocation below. Include in your figure the appropriate curved arrows showing how one contributor is converted to the next.

b) Fill in the blanks: the conjugated pi system in this carbocation is composed of ______ 2p orbitals sharing ________ delocalized pi electrons.
- Answer
-
a)
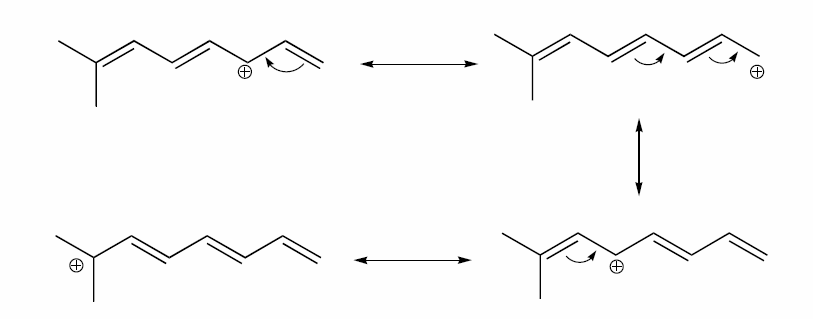
b) The conjugated pi system in this carbocation is composed of seven p orbitals containing six delocalized pi electrons.
Draw the major resonance contributor of the structure below. Include in your figure the appropriate curved arrows showing how you got from the given structure to your structure. Explain why your contributor is the major one. In what kind of orbitals are the two lone pairs on the oxygen?
Solution
In the structure above, the carbon with the positive formal charge does not have a complete octet of valence electrons. Using the curved arrow convention, a lone pair on the oxygen can be moved to the adjacent bond to the left, and the electrons in the double bond shifted over to the left (see the rules for drawing resonance contributors to convince yourself that these are 'legal' moves).
The resulting resonance contributor, in which the oxygen bears the formal charge, is the major one because all atoms have a complete octet, and there is one additional bond drawn (resonance rules #1 and #2 both apply). This system can be thought of as four parallel 2p orbitals (one each on C2, C3, and C4, plus one on oxygen) sharing four pi electrons. One lone pair on the oxygen is in an unhybridized 2p orbital and is part of the conjugated pi system, and the other is located in an sp2 orbital.
Also note that one additional contributor can be drawn, but it is also minor because it has a carbon with an incomplete octet:
The figure below shows how the negative formal charge on an oxygen (of an enol) can be delocalized to the carbon indicated by an arrow. More resonance contributors can be drawn in which negative charge is delocalized to three other atoms on the molecule.
a) Circle these atoms that can also have a resonance structure with a negative charge.
b) Draw the two most important resonance contributors for the enolate ion.
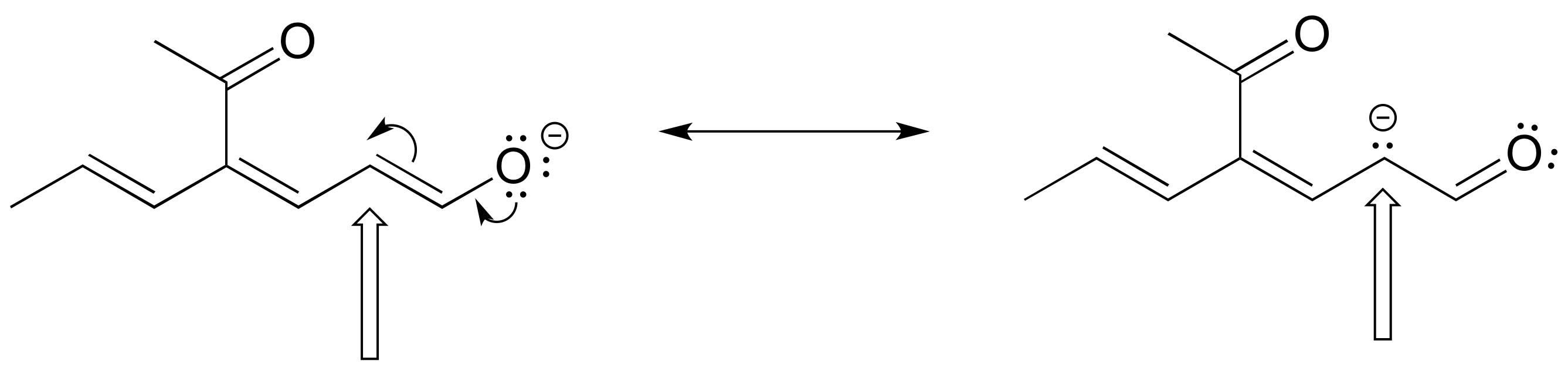
- Answer
-
The two major contributors are those in which the negative formal charge is located on an oxygen rather than on a carbon.
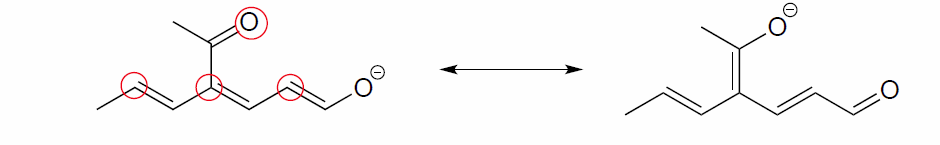
Exercise 2.6.5:
a) Draw three additional resonance contributors for the carbocation below. Include in your figure the appropriate curved arrows showing how one contributor is converted to the next.

b) Fill in the blanks: the conjugated pi system in this carbocation is composed of ______ 2p orbitals sharing ________ delocalized pi electrons.
Exercise 2.6.6: Draw the major resonance contributor for each of the anions below.
c) Fill in the blanks: the conjugated pi system in part (a) is composed of ______ 2p orbitals containing ________ delocalized pi electrons.
Exercise 2.6.7: The figure below shows how the negative formal charge on the oxygen can be delocalized to the carbon indicated by an arrow. More resonance contributors can be drawn in which negative charge is delocalized to three other atoms on the molecule.
a) Circle these atoms.
b) Draw the two most important resonance contributors for the molecule.

A word of advice
Becoming adept at drawing resonance contributors, using the curved arrow notation to show how one contributor can be converted to another, and understanding the concepts of conjugation and resonance delocalization are some of the most challenging but also most important jobs that you will have as a beginning student of organic chemistry. If you work hard now to gain a firm grasp of these ideas, you will have come a long way toward understanding much of what follows in your organic chemistry course. Conversely, if you fail to come to grips with these concepts now, a lot of what you see later in the course will seem like a bunch of mysterious and incomprehensible lines, dots, and arrows, and it will be difficult to be successful in organic chemistry.
References
- Petrucci, Ralph H., et al. General Chemistry: Principles and Modern Applications. New Jersey: Pearson Prentice Hall, 2007. Print.
- Ahmad, Wan-Yaacob and Zakaria, Mat B. "Drawing Lewis Structures from Lewis Symbols: A Direct Electron Pairing Approach." Journal of Chemical Education: Journal 77.3: n. pag. Web. March 2000. Link to this journal: pkukmweb.ukm.my/~mbz/c_penerb...83%29/p329.pdf
Problems
- True or False, The picture below is a resonance structure?
- Draw the Lewis Dot Structure for SO42- and all possible resonance structures. Which of the following resonance structure is not favored among the Lewis Structures? Explain why. Assign Formal Charges.
- Draw the Lewis Dot Structure for CH3COO- and all possible resonance structures. Assign Formal Charges. Choose the most favorable Lewis Structure.
- Draw the Lewis Dot Structure for HPO32- and all possible resonance structures. Assign Formal Charges.
- Draw the Lewis Dot Structure for CHO21- and all possible resonance structures. Assign Formal Charges.
- Draw the Resonance Hybrid Structure for PO43-.
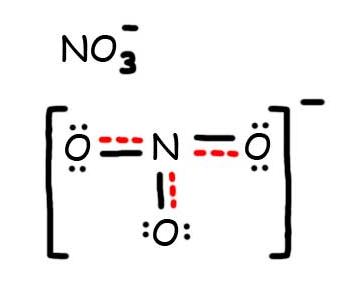
- Draw the Resonance Hybrid Structure for NO3-.
Answers
1. False, because the electrons were not moved around, only the atoms (this violates the Resonance Structure Rules).
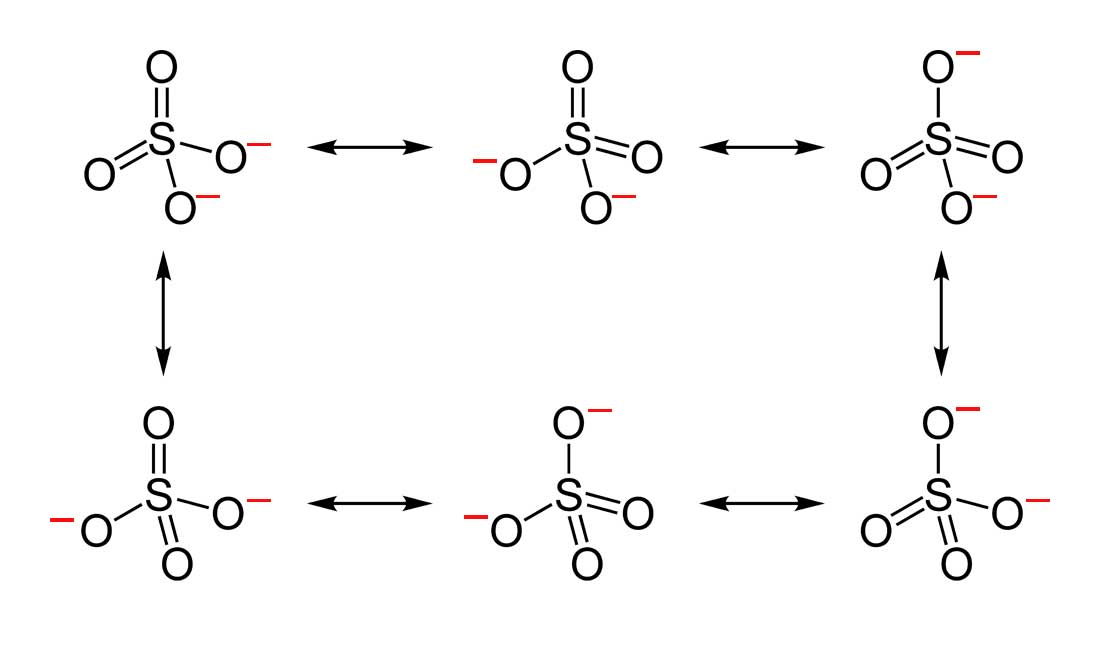
2. Below are the all Lewis dot structure with formal charges (in red) for Sulfate (SO42-). There isn't a most favorable resonance of the Sulfate ion because they are all identical in charge and there is no change in Electronegativity between the Oxygen atoms.
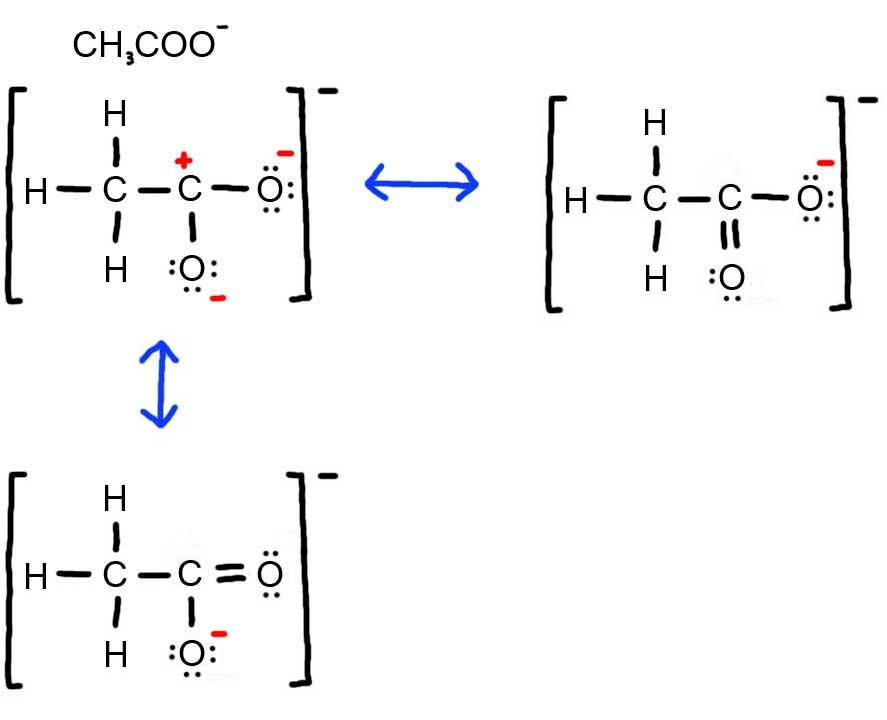
3. Below is the resonance for CH3COO-, formal charges are displayed in red. The Lewis Structure with the most formal charges is not desirable, because we want the Lewis Structure with the least formal charge.
4. The resonance for HPO32-, and the formal charges (in red).
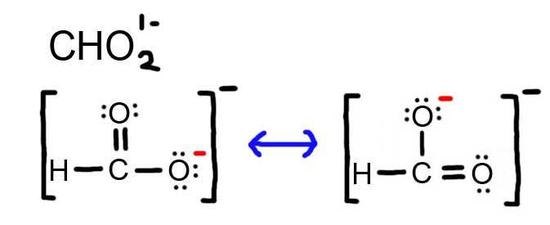
5. The resonance for CHO21-, and the formal charges (in red).
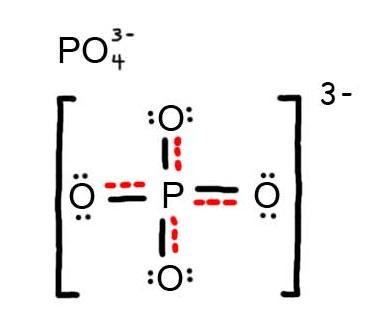
6. The resonance hybrid for PO43-, hybrid bonds are in red.
7. The resonance hybrid for NO3-, hybrid bonds are in red.