22.1: Keto-Enol Tautomerism
- Page ID
- 36412
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- write an equation to illustrate keto‑enol tautomerism.
- write a detailed mechanism for acid‑catalyzed keto‑enol tautomerism.
- write a detailed mechanism for base‑catalyzed keto‑enol tautomerism.
- draw the structure of the enol form of a given carbonyl compound.
Make certain that you can define, and use in context, the key terms below.
- enol
- keto
- tautomerism
- tautomers
- enolate ion
Keto‑enol tautomerism was first introduced in Section 9.4, in the discussion of the hydration of alkynes. The subject was raised again in the chapter entitled A Preview of Carbonyl Compounds, during the brief overview of the alpha‑substitution reactions of carbonyl compounds. You may wish to review these sections before proceeding.
Often, the position of a carbon atom near a carbonyl group is designated using Greek letters. The atom adjacent to the carbonyl is alpha, the next removed is beta and so on. The carbon in the carbonyl group is used as reference point and is not assigned a Greek letter. Likewise, hydrogens bare the same Greek letter as the carbon atoms to which they are attached. α-Hydrogens are bonded to α-carbons and β-hydrogens are bonded to β-carbons etc.
The presence of α-hydrogens in a molecule provides the possibility of certain chemical reactions, which will be discussed in this chapter and in Chapter 23. Because of this, the ability to identify α-hydrogens is an important skill. As shown below, pentanal has two α-hydrogens. Note that aldehyde hydrogens are not given a Greek letter, they are simply referred to as an aldehyde hydrogen.
α-hydrogens, which are attached to a carbon directly adjacent to a carbonyl group, display unusual acidity. This is almost exclusively due to the resonance stabilization of the carbanion conjugate base, called an enolate, as illustrated in the diagram below. The effect of the the stabilizing C=O is seen when comparing the pKa for the α-hydrogens of aldehydes (~16-18), ketones (~19-21), and esters (~23-25) to that of a typical alkyl C-H bond (~40-50).
Indicate any α-hydrogens contained in the following molecules:
Solution
Keto-enol Tautomerization
Because of the acidity of α-hydrogens, many carbonyl containing compounds undergo a proton-transfer equilibrium called tautomerism. Tautomers are readily interconverted constitutional isomers, usually distinguished by a different location for an atom or a group. Because tautomers involve the rearrangement of atoms, they are distinctly different than resonance forms, which only differ in the position of bonds and lone pair electrons. This discussion focuses on carbonyl groups with α-hydrogens, which undergo keto-enol tautomerism. Keto implies that the tautomer contains a carbonyl bond while enol implies the presence of a double bond and a hydroxyl group.
The keto-enol tautomerization equilibrium is dependent on stabilization factors of both the keto tautomer and the enol tautomer. For simple carbonyl compounds under normal conditions, the equilibrium usually strongly favors the keto tautomer (acetone, for example, is >99.999% keto tautomer). The keto tautomer is preferred because it is usually more stable than the enol tautomer by about 45–60 kJ/mol, which is mainly due to the C=O double bond (-749 kJ/mol) being stronger than the C=C double bond (-611 kJ/mol). Because ketones have two alky groups donating electron density into the carbonyl carbon, they tend to be more stable and therefore less apt to form the enol tautomer than aldehydes. For example, propanal is 1000 times more likely to be in its enol tautomer than acetone. With carboxylic acid derivatives, the leaving group tends to stabilize the carbonyl through electron donation which makes the formation of the enol tautomer much less likely. In general, ketones are over 100,000,000 times more likely to be in an enol tautomer form than esters.
Aldehydes and symmetrical ketones typically only have one possible enol tautomer while asymmetrical ketones can have two or more. The preferred enol tautomer formed can be often be predicted by considering effects which can stabilize alkenes, such as conjugation and alkyl group substitution. The asymmetrical ketone, 2-methylcyclohexanone has two possible enol tautomers. Of the two tautomers, 2-methyl-1-cyclohexen-1-ol, is the more stable and therefore preferred due to the presence of an additional alkyl substituent. Likewise, 1-phenyl-1-propen-2-ol is the more stable enol tautomer of 1-phenyl-2-propanone due to conjugation with the phenyl ring.
1,3-Dicarbonyls
In certain cases additional stabilizing effects allow the enol tautomer to be preferred in the tautomerization equilibrium. In particular, the 1,3 arrangement of two carbonyl groups can work synergistically to stabilize the enol tautomer, increasing the amount present at equilibrium. The diketone, 2,4-pentanedione, is in its enol form 85% of the time under normal conditions. The positioning of the carbonyl groups allows for the formation of a stabilizing intramolecular hydrogen bond between the hydroxyl group of the enol and the carbonyl oxygen. The alkene group of the enol tautomer is also conjugated with the carbonyl double bond which provides additional stabilization. Both of these stabilizing effects are not possible in the keto tautomer.
Another effect which can stabile an enol tautomer is aromaticity. When considering the molecule 2,4-cyclohexadienone, the enol tautomer is the aromatic molecule phenol. The stabilization gained by forming an aromatic ring is sufficient to make phenol the exclusive tautomer present in the equilibrium.
Please all of the possible enol tautomers for the following compounds. If more than one is possible then indicate which is the most stable and why.
a)
b)
c)
Solution
a)
b)
c)
Mechanism for Catalyzed Keto-Enol Tautomerization
The enol tautomer has valuable nucleophilic characteristics. In neutral media, tautomerization is slow but it can be speed up by catalysis with acids or bases. Both pathways involve two separate proton transfer steps. Because enols are a key reactive intermediate, these mechanistic steps will be used repeatedly in later reactions. The following mechanistic steps represent the continuous interconversion between the keto and enol tautomers.
Overall Process
Acidic Conditions
Keto Tautomer → Enol Tautomer
In the first step, the carbonyl oxygen is protonated by an acid to form an intermediate oxonium ion. A base removes an α-hydrogen during the second step forming a double bond by an E2 type reaction. This causes the pi electrons of the protonated carbonyl to move to the oxygen to form the hydroxyl group of the enol product and regenerating the acid catalyst.
1) Protonation of the carbonyl to form an oxonium ion
2) Deprotonation of an α-hydrogen to form an enol
Enol Tautomer → Keto Tautomer
First, one of the lone pairs of electrons on the enol oxygen moves to form a pi bond with the adjacent carbon to create a oxonium ion. This also causes the pi bond electrons from the enol double bond to attack the electrophilic H+ provided by acid catalyst forming a C-H bond in the α-position. This produced oxonium ion intermediate is subsequently deprotonated to form the neutral ketone and regenerate the acid catalyst.
1) Protonation at the α-carbon
2) Deprotonation
Under Basic Conditions
Keto Tautomer → Enol Tautomer
In the first step, a base removes an α-hydrogen from a carbonyl containing compound to form an alkene by an E2 like process. The causes the pi electrons of the carbonyl bond to move onto the carbonyl oxygen to form an enolate anion. The oxygen of the enolate anion is protonated in the second step to create a neutral enol and regenerate the base catalyst.
1) Deprotonation of a α-hydrogen to form an enolate ion
2) Protonation the enolate ion to form an enol
l
Enol Tautomer → Keto Tautomer
The mechanistic return to the keto tautomer begins with deprotonation of the hydroxyl hydrogen to produce an enolate ion. Then lone pair electrons from the enolate anion attack an electrophilic H+ through conjugation with the double bond. This simultaneously forms the carbonyl double bond, adds an alpha hydrogen, and regenerates the base catalyst.
1) Deprotonation of the enol hydrogen
2) Protonation of the α-carbon
Biological Enol Forming Reactions
One very important family of isomerase enzymes catalyzes the shifting of a carbonyl group in sugar molecules using a process called an carbonyl isomerization. Carbonyl isomerization often involves converting between a ketose and an aldose. (recall that the terms ketose and aldose refer to sugar molecules containing ketone and aldehyde groups, respectively).
Carbonyl Isomerization
Mechanism
Carbonyl isomerization can only occur if there is an OH group adjacent to the carbonyl. Isomerization forms an ene-diol intermediate which has both OH hydrogens available to be removed to form a carbonyl. If the hydrogen from the original OH group is removed a new carbonyl bond is formed.
Carbonyl isomerization is involved in the metabolism of carbohydrates (starches and sugars) to their eventual conversion to CO2 and H2O. First, starches are broken down into glucose in the digestive tract. In the cells, the first step of the glycolysis pathway involves an enzyme converting glucose to glucose-6-phosphate. This is followed by the enzyme-catalyzed tautomerization of glucose-6-phosphate (an aldose) to fructose-6-phosphate (a ketose) through an enediol intermediate. Notice how the carbonyl has moved from the 1-carbon (terminal) to the 2-carbon.
Exercises
1) Draw the enol forms of the following molecules
- 4-methylcyclohexanone
- Ethyl thioactetate
- Methyl acetate
- Butanal
- 1-phenyl-2-butanone
2) How many α-hydrogens do each of the molecules from the previous question have? Label them.
3) Draw all of the mono-enol forms for the following molecule. Which ones are most stable? Why?
4) Under normal conditions cyclohexanone exists in the enol tautomer in a much higher percentage than acetone. Explain.
5) The 1,3-dicarbonyl shown below is only as acidic as acetone and does not form a detectable amount of the enol tautomer. Please explain.
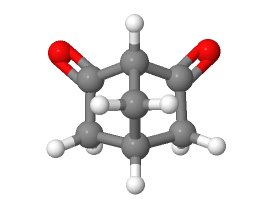
6) For the following compound please identify the most acidic hydrogen atom. Remember that for an enolate conjugate base to be stabilized through conjugation, the lone pair electrons need to be contained in a p orbital which is parallel to the p orbitals which form the C=O pi bond.
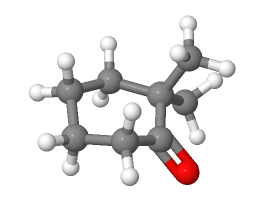
7) Nonconjugated ß, gamma-unsaturated ketones, such as 2-hexen-4-one, can be converted to their α, ß-unsaturated isomers by treatment with an acid catalyst. Please propose a mechanism for this isomerization.
Solutions
1)
(a)
(b)
(c)
(d)
(e)
2)
(a)
(b)
(c)
(d)
(e)
3)
Additional resonance forms stabilizes this enol.
This enol has fewer resonance forms and is therefore less stable.
4) The enol tautomer of cyclohexanone has more alky substituents than the enol tautomer of acetone. This makes the double bond of the enol double bond of cyclohexanone more stable and easier to form.
5) In many situations, the enol tautomer fo the a 1,3-dicarbonyl is preferred in the tautomerization equilibrium due to stabilizing effects created by the second carbonyl. However, in this case the alpha hydrogen used to create the enol tautomer is attached to a brigehead carbon of a bicyclic ring system. The positioning of the enol and the carbonyl prevents the formation of a stabilizing intramolecular hydrogen bond between the hydroxyl group of the enol and the carbonyl oxygen. Also, the inherent steric restrictions of a bicyclic ring system prevents the formation of a double bond using a bridgehead carbon. Instead, an enol tautomer of the molecule would be expected to form outside the 1,3-dicarbonyl. This enol lacks the stabilizing effects typical of a 1,3-dicarbonyl, so it is not preferred in the tautomerization equilibrium.
6) The presence of two methyl groups on one of the α-carbons of the carbonyl means that the two hydrogens on the other α-carbon may be deprotonated to form an enolate. When the indicated axial hydrogen is deprotonated the p-orbital formed is parallel with the carbonyl p-orbitals and can participate in overlap. If the equatorial hydrogen were to be deprotonated, the p-orbital formed would be perpendicular with the carbonyl p-orbitals and prevented from participating in overlap.
7)


