22.S: Carbonyl Alpha-Substitution Reactions (Summary)
- Page ID
- 207031
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
Concepts & Vocabulary
- Four common types of reactions involving carbonyl reactions: 1) nucleophilic addition; 2) nucleophilic acyl substitution; 3) alpha substitution; 4) carbonyl condensations. The first two were previously discussed and the second two involve the properties of the carbon directly adjacent to the carbonyls, α carbons.
- Alpha-substitution reactions results in the replacement of an H attached to the alpha carbon with an electrophile.
- The nucleophile in these reactions are new and called enols and enolates.
- In this chapter, the focus is on α substitutions reactions with aldehydes and ketones.
22.1 Keto-Enol Tautomerization
- Greek letters are used to denote the carbon atoms near carbonyls.
- The carbon in the carbonyl is the reference point and the alpha carbon is adjacent to the carbonyl carbon.
- Hydrogen atoms attached the these carbons denoted with Greek letters will have the same designation, so an alpha hydrogen is attached to an alpha carbon.
- Aldehyde hydrogens not given Greek leters.
- α hydrogens display unusual acidity, due to the resonance stabilization of the carbanion conjugate base, called an enolate.
- Tautomers are readily interconverted constitutional isomers, usually distinguished by a different location for an atom or a group, which is different than resonance.
- The tautomerization in this chapter focuses on the carbonyl group with alpha hydrogen, which undergo keto-enol tautomerism.
- Keto refers to the tautomer containing the carbonyl while enol implies a double bond and a hydroxyl group present in the tautomer.
- The keto-enol tautomerization equilibrium is dependent on stabilization factors of both the keto tautomer and the enol tautomer, though the keto form is typically favored for simple carbonyl compounds.
- The 1,3 arrangement of two carbonyl groups can work synergistically to stabilize the enol tautomer, increasing the amount present at equilibrium.
- The positioning of the carbonyl groups in the 1,3 arrangement allows for the formation of a stabilizing intramolecular hydrogen bond between the hydroxyl group of the enol and the carbonyl oxygen as well as the alkene group of the enol tautomer is also conjugated with the carbonyl double bond which provides additional stabilization.
- Aromaticity can also stabilize the enol tautomer over the keto tautomer.
- Under neutral conditions, the tautomerization is slow, but both acid and base catalysts can be utilized to speed the reaction up.
- Biological enol forming reactions use isomerase enzymes to catalyze the shifting of a carbonyl group in sugar molecules, often converting between a ketose and an aldose in a process called carbonyl isomerization.
22.2 Reactivity of Enols: The Mechanism of Alpha-Substitution Reactions
- The oxygen of the enol donates electron density to the double bond making it more electron rich and thus more reactive than a typical alkene.
- The mechanism starts with an acid-catalyzed tautomerization to form an enol.
- The double bond is then able to act as a nucleophile and attack an electrophile.
- The final product is an alpha-substituted carbonyl after the deprotonation of the carbonyl to also regenerate the acid-catalyst.
- The enol formed has planar geometry, which means the electrophile can attach on the top or bottom of the alpha-carbon.
- A racemic mixture can result if a sterocenter is created at the site of substitution.
22.3 Alpha Halogenation of Aldehydes and Ketones
- Aldehydes and ketons can undergo a substitution of an alpha hydrogen to a halogen.
- An acid-catalyzed tautomerization starts the mechanism followed by the enol attacking molecular halogens.
- The nucleophile in this reaction is the enol and the electrophile is the halogen.
- Mechanistic studies showed that the reaction was a first-order in the ketone.
- The halogen is part of a fast step after the rate-determing step.
- The formation of an enol intermediate was provided using a reaction called deuterium exchange.
- Due to the acidic nature of α hydrogens they can be exchanged with deuterium by reaction with the isotopic form of water, D2O.
- The mechanism for deuterium exchange is virtually the same as that of keto-enol tautomerism under acidic conditions, the difference being a deuterium is placed in the α-position.
- Both alpha halogenation and deuterium exhange reactions were found to have a common intermediate involved in the rate determining step of their mechanism, an enol.
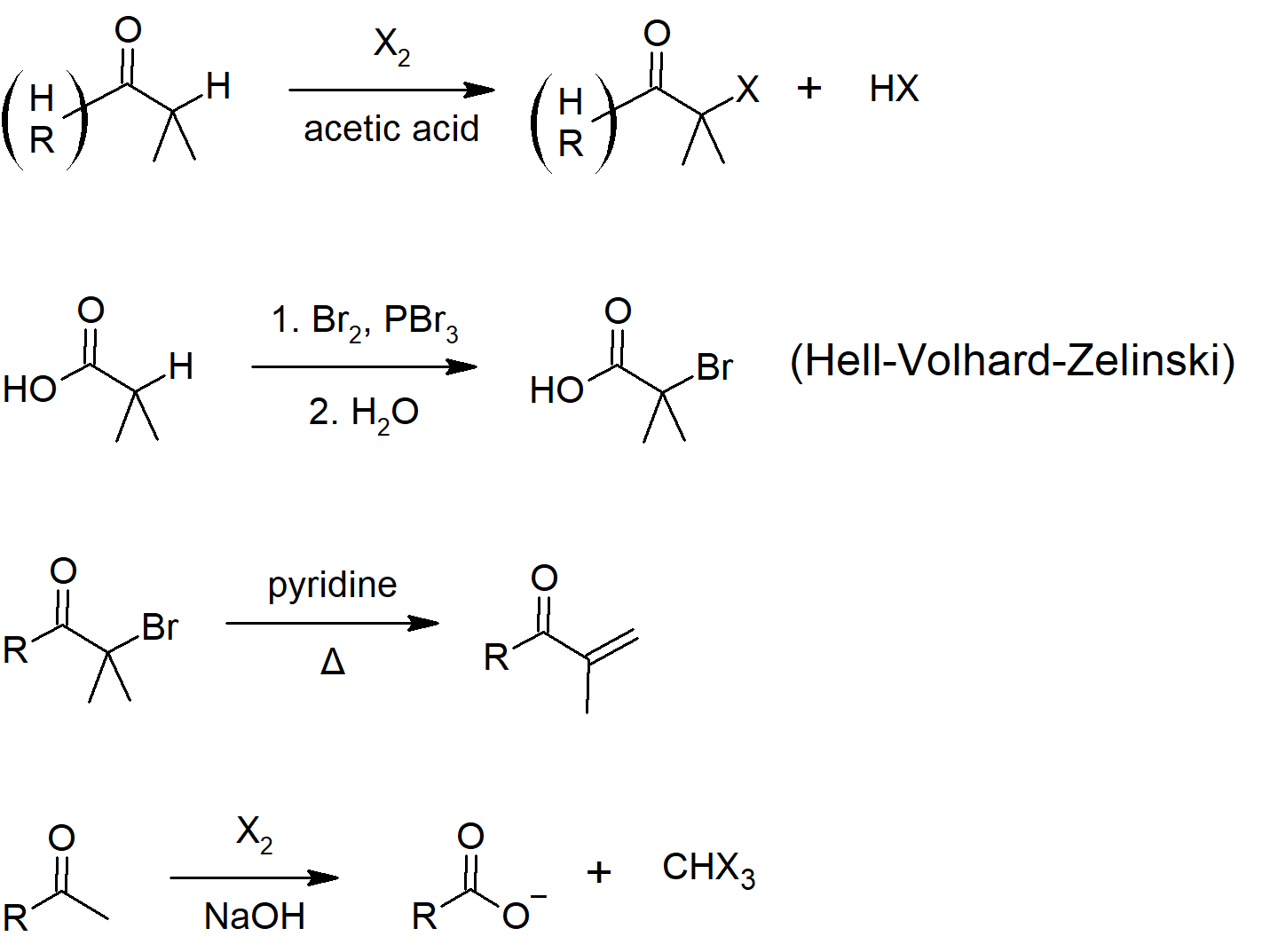
22.4 Alpha Bromination of Carboxylic Acids
- The α-bromination of some carbonyl compounds, such as aldehydes and ketones, can be accomplished with Br2 under acidic conditions, but the reaction will generally not occur with more stable carboxylic acid derivatives.
- Carboxylic acids do not enolize to a sufficient extent since the carboxylic acid proton is preferably removed before an α-hydrogen.
- Carboxylic acids, can be brominated in the α position with a mixture of Br2 and phosphorus tribromide (PBr3) in what is called the Hell-Volhard-Zelinskii reaction.
- α-Bromo carboxylic acids are extremely useful synthetic intermediates because the halogen is highly reactive towards SN2 reaction.
- Reaction of a α-bromo carboxylic acids with an excess of ammonia provides α-amination, which is a possible route to amino acids.
22.5 Acidity of Alpha Hydrogen Atoms: Enolate Ion Formation
- α hydrogens are weakly acidic because the conjugate base, called an enolate, is stabilized though conjugation with the π orbitals of the adjacent carbonyl.
- The enolate has two resonance structures to contribute to the resonance hybrid.
- While α-hydrogens are weakly acidic, typical strong bases such as hydroxide or alkoxide are only capable of forming the enolate ion in very low concentrations.
- To achieve complete deprotonation of aldehyde or ketone reactants to their enolate conjugate bases, a very strong base such as LDA (lithium diisopropylamide) must be used.
- Hydrogen atoms with two or more adjacent carbonyl groups are more acidic than typical α hydrogens, such as β-diketones, β-keto-esters, and β-diesters, which create enolates that are stabilized through additional resonance forms which share the negative charge with multiple carbonyl carbons.
- The acidity of these compounds is increased to the point where typical strong bases such as hydroxide and alkoxide can be used to form the enolate.
22.6 Reactivity of Enolate Ions
- Enolates are better nucleophlies than enols.
- α-hydrogen containing compounds can be completely converted to an enolate by reaction with a strong base whereas enols can only be created in small amounts through manipulating their equilibrium.
- Either the C of the O reactive site in an enolate may act as a nucleophile depending on the reaction conditions, but reactions involving the nucleophilic α-carbon are more common, partially due to the thermodynamic stability of the C=O bonds in the final products.
- An enolate reacts rapidly with a halogen to produce α-halogenated carbonyl products.
- The α-hydrogens of halogenated carbonyl products are usually more acidic than the corresponding non-halogenated compounds, which promotes polyhalogenated products.
- The Haloform reaction represents a method for the conversion of methyl ketones to carboxylic acids.
- This reaction is considered a base promoted and not base catalyzed because an entire equivalent of base is required for each α-halogenation.
- Deprotonation of an α-hydrogen with hydroxide produces the nucleophilic enolate ion which subsequently reacts with the halogen.
- The increasing acidity of α-halogenated ketone causes this reaction to occur two more times.
- Once formed, the CX3 group attached to the carbonyl can act as a leaving group, eventually produces a haloform (CHCl3, CHBr3, CHI3) and a carboxylate anion.
22.7 Alkylation of Enolate Ions
- Enolates can be alkylated in the alpha position through an SN2 reaction with alkyl halides.
- An α hydrogen is replaced with an alkyl group and a new C-C bond is formed.
- Very strong bases like LDA are often used to fully deprotonate the carbonyl and completely form the enolate.
- Direct alkylations, like all enolate-based reactions, will form a racemic mixture if the alkylated alpha carbon is chiral.
- When an unsymmetrical ketone with two sets of nonequivalent alpha hydrogens is treated with a base, two possible enolates can form.
- The main determinant for which enolate is formed is whether the reaction is under kinetic control (rate) or thermodynamic (equilibrium) control.
- The thermodynamic enolate is formed when the more substituted alpha hydrogen is removed, yielding the more alkyl substituted, therefore the most stable, enolate.
- Formation of the thermodynamic enolate is sterically hindered and is kinetically slow, especially with a bulky base like LDA.
- Kinetic enolates are formed when the less substituted alpha hydrogen is deprotonated, being less sterically hindered allows this alpha hydrogen to be deprotonated faster even though it forms a less thermodynamically stable enolate.
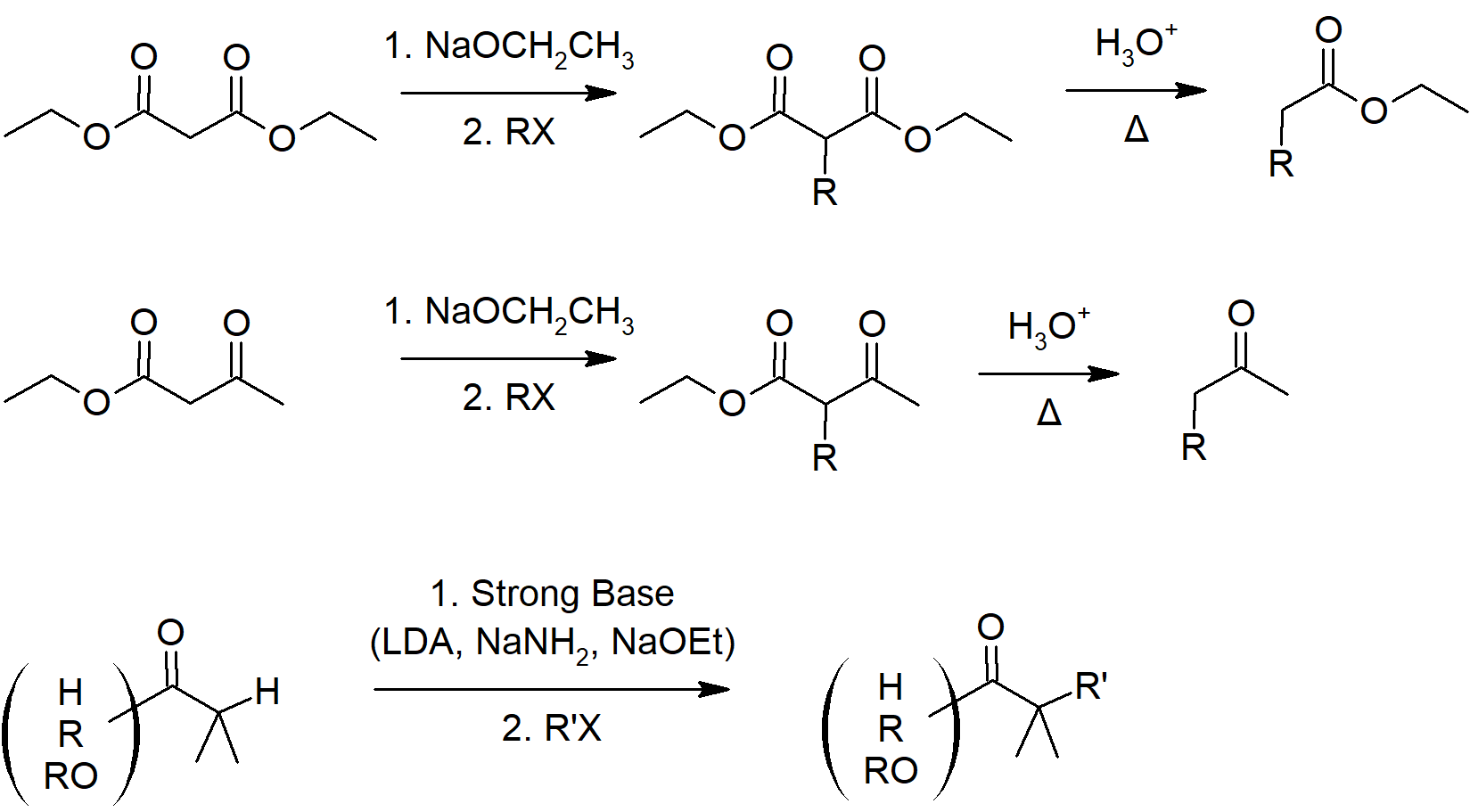
- The malonic ester synthesis is a series of reactions which converts an alkyl halide to a carboxylic acid with two additional carbons.
- The importance of this synthesis pathways is that it allows for the creation of alpha alkylated carboxylic acids which cannot be created by direct alkylation.
- The acetoacetic ester synthesis is a series of reaction which converts alkyl halides into a methyl ketone with three additional carbons.
- This reaction creates an alpha substituted methyl ketone without side-products.
- In retrosynthetic analysis, a C-C bond is broken between the alpha carbon and the beta carbon away from this functionality.
Skills to Master
- Skill 22.1 Determine alpha carbons and alpha hydrogens.
- Skill 22.2 Draw the enols formed from carbonyl derivatives.
- Skill 22.3 Provide the mechanism for the keto-enol tautomerization under neutral, acidic and basic conditions.
- Skill 22.4 Provide the mechanism for the enol-keto tautomerization under neutral, acidic and basic conditions.
- Skill 22.5 Draw the products of halogenation reactions.
- Skill 22.6 Provide the mechanism for halogenation reactions.
- Skill 22.7 Write an equation to illustrate the Hell‑Volhard‑Zelinskii reaction.
- Skill 22.8 Identify the product formed from the reaction of a given carboxylic acid with bromine and phosphorus tribromide.
- Skill 22.9 Explain why the alpha hydrogen of carbonyl compounds are more acidic than a typical hydrogen.
- Skill 22.10 Explain why dicarbonyl protons are more acidic than compounds that contain a single carbonyl.
- Skill 22.11 Provide a mechanism for the haloform reaction.
- Skill 22.12 Provide a mechanism for an alkylation reaction with an enolate.
- Skill 22.13 Provide a mechanism for malonic ester synthesis.
- Skill 22.14 Propose synthesis for alkylated carboxylic acids.
- Skill 22.15 Provide a mechanism for acetoacetic acid synthesis.
- Skill 22.16 Propose a synthesis for alkylated methyl ketones.
Summary of Reactions
Alkylation