17.8: Protection of Alcohols
- Page ID
- 36352
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- explain what is meant by “protecting” a functional group during an organic synthesis.
- describe one common method for protecting the hydroxy group of an alcohol, and give an example of its use (e.g., in the preparation of a Grignard reagent).
Often during the synthesis of complex molecules one functional group in a molecule interferes with an intended reaction on a second functional group on the same molecule. An excellent example is the fact that a Grignard reagent can't be prepared from halo alcohol because the C-Mg bond is not compatible with the acidic -OH group.
When situations like this occurs chemists circumvent the problem by changing the interfering functional group into one that does not interfere with the intended reaction. Chemists call this process protection of a functional group. Functional group protection involves three steps:
- Blocking the interfering functionality by introducing a protecting group.
- Performing the intended reaction.
- Removing the protecting group and reforming the original functional group.
Protecting Alcohols Through the Formation of Trialkylsilyl Ethers
There are several methods for protecting an alcohol, however, the most common is the reaction with a chlorotrialkylsilane, Cl-SiR3 This reactions forms a trialkylsilyl ether, R'O-SiR3. One of the most common reagents is chlorotrimethylsilane [(CH3)3SiCl] which is often used in conjunction with a base, such as triethylamine. The base helps to form the alkoxide anion and remove the HCl produced by the reaction. The produced trimethylsilyl ether is commonly abbreviated -OTMS. This silyl ether no longer has the fairly acidic OH proton as the alcohol group has been changed (protected).
General Reaction
Mechanism
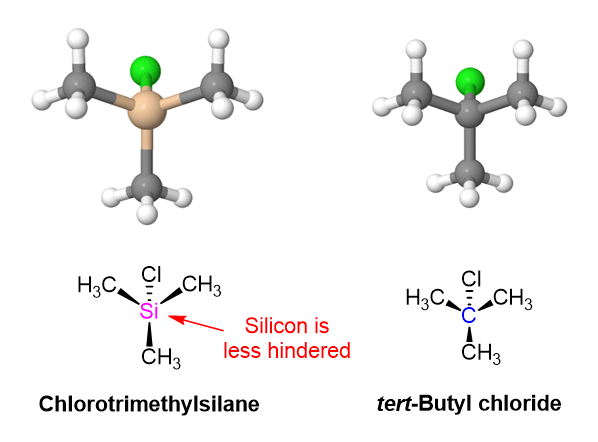
Notice this reaction occurs through an SN2-like mechanism with the alcohol attacking a trialkyl-substitued silicon atom. Normally, an SN2 reaction would not be viable for the analogous tert-butyl chloride due to the steric hindrance of the tertiary electrophilic carbon. Being a third-row atom, silicon is larger and forms longer bonds that carbon. The Si-C bonds of chlorotrimethylsilane are 195 pm compared to the 154 pm C-C bonds or tert-butylchloride. The three methyl groups attached to silicon are more spread out and offer less steric hindrance so the SN2-like reaction can occur.
Deprotection
Trimethylsilyl ethers, like most other ethers, are relatively unreactive towards many reagents such as oxidizing agents, reducing agents, or Grignard reagents. However, the TMS ether protecting group can be removed by reaction with an aqueous acid or the fluoride ion (F-) to regenerate the alcohol. Common sources of the fluoride ion are lithium fluoride (LiF) and tetrabutylammoniumfluoride (TBAF) [(CH3CH2CH2CH2)4NF]. This step is commonly referred to as a deprotection.
Utilizing a Protecting Group for a Grignard Reaction
The problem posed at the beginning of this section can be solved through the use of a TMS protecting group. Once the alcohol is converted into a TMS ether the acidic hydrogen will no longer be present and a Grignard reagent can be formed.
1) Protection of the Alcohol
2) Form the Grignard Reagent
3) Perform the Grignard Reaction
4) Deprotection
Exercises
Propose a multiple-step synthesis to transform 4-bromo-1-butanol into 2-methylhexane-2,6-diol.
- Answer
-
Contributors and Attributions
- Layne Morsch (University of Illinois Springfield)
- James Kabrhel (University of Wisconsin - Green Bay, Sheboygan Campus)


