21.6: Chemistry of Esters
- Page ID
- 36403
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
- discuss the wide occurrence of esters in nature, and their important commercial uses, giving one example of an ester linkage in nature, and one example of a commercially important ester.
- write an equation to describe the hydrolysis of an ester under acidic or basic conditions.
- identify the products formed from the hydrolysis of an given ester.
- identify the reagents that can be used to bring about ester hydrolysis.
- identify the structure of an unknown ester, given the products of its hydrolysis.
- write the mechanism of alkaline ester hydrolysis.
- write the mechanism of acidic ester hydrolysis.
- write an equation to describe the reduction of an ester with lithium aluminum hydride.
- identify the product formed from the reduction of a given ester (or lactone) with lithium aluminum hydride.
- identify the ester, the reagents, or both, that should be used to prepare a given primary alcohol.
- write a detailed mechanism for the reduction of an ester by lithium aluminum hydride.
- identify diisobutylaluminum hydride as a reagent for reducing an ester to an aldehyde, and write an equation for such a reaction.
- write an equation to describe the reaction of an ester with a Grignard reagent.
- identify the product formed from the reaction of a given ester with a given Grignard reagent.
- identify the ester, the Grignard reagent, or both, needed to prepare a given tertiary alcohol.
- write a detailed mechanism for the reaction of an ester with a Grignard reagent.
Make certain that you can define, and use in context, the key terms below.
- lactone
- saponification
Many esters have characteristic aromas and flavours. Some examples are listed below.
Basic structure:
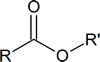
| IUPAC name | R | R′ | Aroma |
|---|---|---|---|
| octyl ethanoate | CH3 | CH3(CH2)6CH2 | orange |
| propyl ethanoate | CH3 | CH3CH2 CH2 | pear |
| 2‑methylpropyl propanoate | CH3CH2 | (CH3)2CHCH2 | rum |
| methyl butanoate | CH3CH2CH2 | CH3 | apple |
| ethyl butanoate | CH3CH2CH2 | CH3CH2 | pineapple |
A “lactone” is a cyclic ester and has the general structure
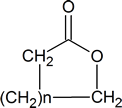
By recognizing that the steps in the acidic hydrolysis of an ester are exactly the same as those in a Fischer esterification (but in the reverse order!), you can again minimize the amount of memorization that you must undertake. The details of both mechanisms can be deduced from the knowledge that both reactions are acid‑catalyzed nucleophilic acyl substitutions.
Esters are present in a many biologically important molecules, which have a wide range of effects including fats, waxes, Vitamin C, Cocaine, Novacaine, oil of wintergreen, and aspirin.
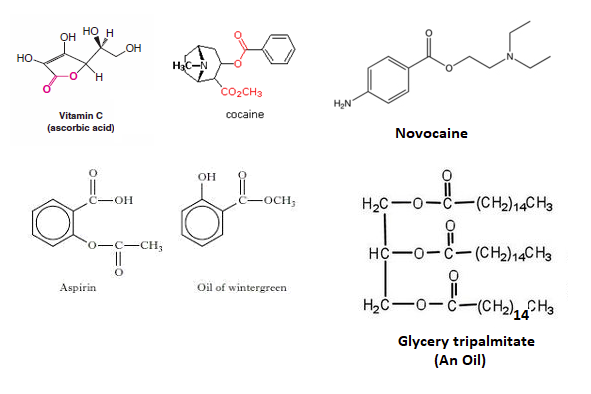
Esters compounds are often the source of the pleasant aromas of many fruits.
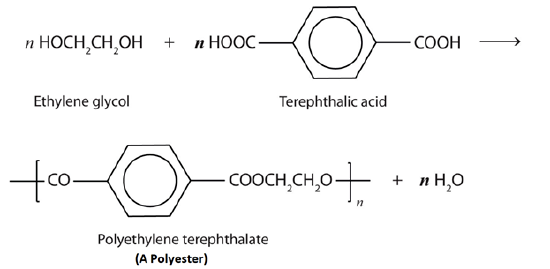
Esters are also present in a number of important commercial and synthetic application. For example, polyester molecules make excellent fibers and are used in many fabrics. A knitted polyester tube, which is biologically inert, can be used in surgery to repair or replace diseased sections of blood vessels. The most important polyester, polyethylene terephthalate (PET), is made from terephthalic acid and ethylene glycol monomers. PET is used to make bottles for water and other beverages. It is also formed into films called Mylar which is used in balloons. Synthetic arteries can be made from PET, polytetrafluoroethylene (PTFE), and other polymers.
Lactones, cyclic esters, have similar reactivity as acyclic esters.
Preparation of Esters
The most versatile method for the preparations of esters is the nucleophilic acyl substitution of of an acid chloride with an alcohol. Acid ahydrides and carboxylic acids can also react with alcohols to form esters but both reactions are limited to formation of simple esters. Esters can also be formed by deprotonating a carboxylic acid to form a carboxylate and then reacting it with a primary alkyl halide using an SN2 reaction.
Reactions of Esters
Esters are one of the more useful functional groups. Their low reactivity makes the easy to work with and they are stable enough to be used as a solvent in organic reactions (ex. ethyl acetate). Esters are still reactive enough to undergo hydrolysis to form carboxylic acids, alcoholysis, to form different esters, and aminolysis to form amides. Also, they can react with Grignard reagents to form 3o alcohols and hydride reagents to form 1o alcohols or aldehydes.
Conversion of Esters to Carboxylic Acids: Hydrolysis
Esters can be cleaved back into a carboxylic acid and an alcohol through reaction with water and a catalytic amount of strong acid. This reaction represents the reverse of the acid catalyzed esterification of a carboxylic acid and an alcohol discussed in Section 21.3. Both the ester formation and cleavage reactions are part of an equilibrium which can be manipulated using Le Chatelier's principle. For ester hydrolysis, the equilibrium is shifted toward carboxylic acid formation by using a large excess of water in the reaction.
General Reaction
Example
Mechanism
Acid catalysis is required during ester hydrolysis due to water being a weak nucleophile. Protonation of the ester carbonyl increases the partial positive charge on the carbonyl carbon increasing its electrophilicity. After protonation, water adds to the carbonyl carbon causing the formation of a tetrahedral alkoxide intermediate. Then a proton transfers to the –OR group, increasing its ability to act as a leaving group. Reforming the carbonyl double bond causes the elimination of an alcohol (HOR) as a leaving group, creating a protonated carboxylic acid. In the last step of the mechanism, water acts as a base, removing a hydrogen, to form a carboxylic acid and regenerating the acid catalyst.
1) Protonation of the carbonyl
2) Nucleophilic attack by water
3) Proton transfer
4) Leaving group removal
5) Deprotonation
Hydrolysis of Lactones
Lactones (Cyclic esters) undergo typical reactions of esters including hydrolysis. Hydrolysis of the lactone under acidic conditions creates a hydroxyacid.
Conversion of Esters to Carboxylic Acids: Saponification
Esters can also be cleaved into a carboxylate and an alcohol through reaction with water and a base. The reaction is commonly called a saponification from the Latin sapo which means soap. This name comes from the fact that soap used to me made by the ester hydrolysis of fats.
Saponification reaction utilize a better nucleophile (hydroxide) and are typically faster than an acid catalyzed hydrolysis. The carboxylation ions produced by saponification are negatively charged and very unreactive toward further nucleophilic substitution which makes the reaction irreversible.
General Reaction
Example
Mechanism
The base-promoted hydrolysis of an ester follows the typical nucleophilic acyl substitution mechanism. A full equivalent of hydroxide anion is used, so the reaction is called base-promoted and not base catalyzed. The mechanism of ester saponification begins with the nucleophilic addition of a hydroxide ion at the carbonyl carbon to give a tetrahedral alkoxide intermediate. The carbonyl bond is reformed along with the elimination of an alkoxide (-OR) leaving group yielding a carboxylic acid. The alkoxide base deprotonates the carboxylic acid to for a carboxylate salt and an alcohol as products.
The last deprotonation step essentially removes the carboxylic acid from the equilibrium which drives the saponification towards completion. Because the carboxylic acid is no longer part of the equilibrium the reaction is effectively irreversible.
1) Nucleophilic attack by hydroxide
2) Leaving group removal
3) Deprotonation
This mechanism is supported by experiments performed using isotopically labeled esters. When the ether-type oxygen of the ester was labeled with 18O, the labeled oxygen showed up in the alcohol product after hydrolysis.
An alternative mechanism would be if the hydroxide participated in an SN2 reaction to create the carboxylate product. If this were to happen the alcohol reaction product would not contain the labeled oxygen.
Ester saponification in biological systems, called hydrolytic acyl substitution reactions, are common. In particular, acetylcholinesterase, an enzyme present in the synapse, catalyzes hydrolysis of the ester group in acetylcholine which is a neurotransmitter that triggers muscle contraction. Like many other hydrolytic enzymes, the acetylcholinesterase reaction proceeds in two phases: first, a covalent enzyme-substrate intermediate is formed when the acyl group of acetylcholine is transferred to an active-site serine on the enzyme (a transesterification reaction). A water nucleophile then attacks this ester, driving off acetate and completing the hydrolysis
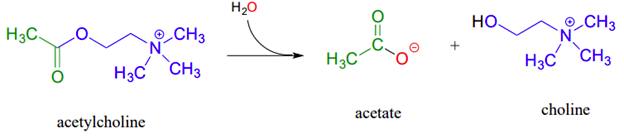
Conversion of Esters to Different Esters: Transesterification
Transesterification is a reaction where an ester is converted to a different ester through reaction with an alcohol. Because there is typically very little difference in stability between both esters, the equilibrium constant of this reaction is usually near one. Using a large excess of the reactant alcohol or removing the alcohol side-product can push the reaction equilibrium towards the products by Le Chatelier’s principal. Transesterifications also shows that great care should be taken when an ester containing compound is used in a reaction involving an alcohol.
General Reaction
Example
Mechanism in Basic Conditions
The reaction follows the basic mechanism of a nucleophilic acyl substitution. The alkoxide leaving group of the ester is replace by an incoming alkoxide nucleophile creating a different ester.
1) Nucleophilic attack by an alkoxide
2) Leaving group removal
Mechanism in Acidic Conditions
Protonation allows the alcohol reactant to add to the ester carbonyl. Proton transfer to the ester's alkoxy group increases it ability to act as a leaving group. Reforming the C=O carbonyl bond removes the leaving group and subsequent deprotonation by water forms the ester product.
1) Protonation of the carbonyl
2) Nucleophilic attack on the carbonyl
3) Proton transfer
4) Removal of the leaving group
5) Deprotonation
Conversion of Ester to Amides: Aminolysis
It is possible to convert esters to amides through direct reaction with ammonia or amines. However, these reactions are not commonly used because the formation of an amide using an acid chloride is a much simpler reaction.
Conversion of Esters to 1o Alcohols: Hydride Reduction
Esters can undergo hydride reduction with LiAlH4 to form two alcohols. The alcohol derived from the acyl group of the ester will be 1o and is typically considered the main product of the reaction. The other alcohol is derived from the ester’s alkoxy group and is typically considered a side-product of the reaction. Note! Sodium borohydride (NaBH4) is not a reactive enough hydride agent to reduce esters or carboxylic acids. In fact, NaBH4 can selectively reduce aldehydes and ketones in the presence of ester functional groups.
General Reaction
Predicting the Products of a Hydride Reduction
There are three major changes in bonding during this reaction: 1) The –OR leaving group is removed from the ester. 2) The C=O carbonyl bond is converted to a C-O-H, an alcohol. 3) Two C-H bonds are formed as two of the hydride nucleophiles are added to the original carbonyl carbon of the ester.
Example
Mechanism
The mechanisms for the hydride reduction of esters is analogous to the hydride reduction of carboxylic acids. Nucleophilic acyl substitution replaces the –OR leaving group in ester with a hydride nucleophile to form an aldehyde intermediate. Because aldehydes are more reactive than esters, they rapidly undergo a second nucleophilic hydride addition to form a tetrahedral alkoxide intermediate. An acid work-up protonates the alkoxide to create a 1o alcohol.
1) Nucleophilic attack by the hydride
2) Leaving Group Removal
3) Nucleopilic attack by the hydride anion
4) The alkoxide is protonated
Conversion of Esters to Aldehydes: Hydride Reduction
Much like acid chlorides, esters can be converted to aldehydes using the weaker reducing reagent diisobutylaluminum hydride (DIBALH). As shown above, an aldehyde intermediate is produced after an ester undergoes nucleophilic acyl substitution with a hydride. When DIBALH is used as the hydride source, the aldehyde does not react further and is isolated as the product of the reaction. The reaction is usually carried out at -78 oC to help isolate the aldehyde product.
General Reaction
Example
Conversion of Esters to 3o Alcohols: Grignard Reagents
Addition of Grignard reagents converts esters to two alcohols, one 3o alcohols (main product) and one 1o alcohol (considered a side product). The Grignard reagent adds to the ester twice, once during a nucleophilic acyl substitution to form a ketone intermediate then again during a nucleophilic addition to form the 3o alcohol product. Overall, during this reaction two C-C bonds are formed on the ester’s original carbonyl carbon.
General Reaction
Predicting the Products of a Grignard Reaction
Example
Mechanism
In the first two steps of the mechanism, the OR leaving group from the ester is replaced by the R group from the Grignard reagent through a nucleophilic acyl substitution. This forms a ketone intermediate which is not isolated because ketones, which are more reactive than esters, rapidly undergo nucleophilic addition with a second equivalent of the Grignard reagent to form an alkoxide intermediate. An acid work-up protonates the alkoxide to form the 3o alcohol product.
1) Nucleophilic Attack
2) Leaving group removal
3) Nucleophilic attack
4) Protonation
How could the following molecule be made using a Grignard reagent and an ester?
Solution
The key bond breaks for this example are two C-C sigma bonds between the carbonyl carbon and two alpha carbons. Reactions with esters involve a double addition of the Grignard reagent so the fragments removed must be the same. In this example, the C-C bonds involving the two methyl groups are broken. Breaking these bonds separate the target molecule into the required starting materials. The fragment which contains the alcohol carbon forms a C=O carbonyl bond and gains an –OR to become an ester. The R group of the ester is largely unimportant to the overall reaction and is typically a methyl or ethyl group. The alkyl fragments gain MgBr to form a Grignard reagent. Remember that the Grignard reagent only contains one alkyl fragment.
Exercises \(\PageIndex{1}\)
1) Why is the alkaline hydrolysis of an ester not a reversible process? Why doesn't the reaction with a hydroxide ion and a carboxylic acid produce an ester?
2) Draw the product of the reaction between the following molecule and LiAlH4, and the product of the reaction between the following molecule and DIBAL.
3) How might you Prepare the following molecules from esters and Grignard reagents?
(a)
(b)
(c)
- Answers
-
1) The reaction between a carboxylic acid and a hydroxide ion is an acid base reaction, which produces water and a carboxylate anion.
2)
3)
(a)
(b)
(c)

