10.2: Alkaloid Based Reactions
- Page ID
- 168831
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Conjugate Addition Reactions
Cinchona alkaloids are a large class of compounds extracted from the bark of homonym trees cultivated in equatorial climatic zones, between Bolivian and Venezuelan Andes, and Indonesia. In the extract of the bark are present more than 30 alkaloids (5-15% w/w). Four of them represent 50% of all the alkaloids such as quinine ( QN ), quinidine ( QD ), cinchonidine ( CD ) and cinchonine ( CN ) (Scheme \(\PageIndex{1}\)).
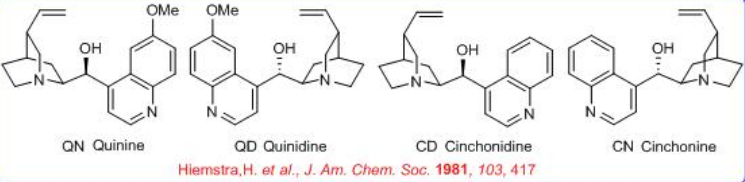
QN is the most well known alkaloid and used as the anti-malarial drug of choice for over 400 years until chloroquine discovered, while QD is used as an anti-arrhythmic agent. In chemistry, all these compounds ( QN , QD , CD and CN) are used as cheap chiral source. These molecules activate the nucleophile by enamine and carbanion formation, and electrophile via hydrogen bond.
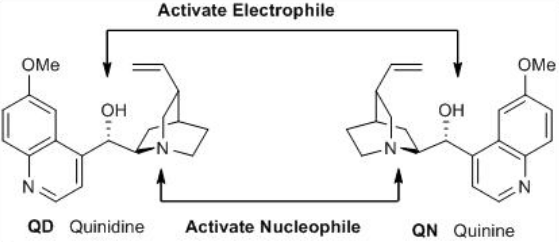
These compounds are diastereomers having five stereogenic centers and the chiral quinuclidinyl nitrogen is the most important as it is responsible of the direct transfer of chirality during catalysis. Quinine vs quinidine and cinchonidine vs cinchonine have opposite absolute configuration this means that very often these pairs of diastereomers act as enantiomers at C-9 position. Furthermore, the C-9 OH group acts as Brønsted acid. So acid and base coexist in these molecules, and thus, it is possible to activate both the nucleophile and the electrophile simultaneously to use as bifunctional organocatalysts (Scheme \(\PageIndex{2}\)).
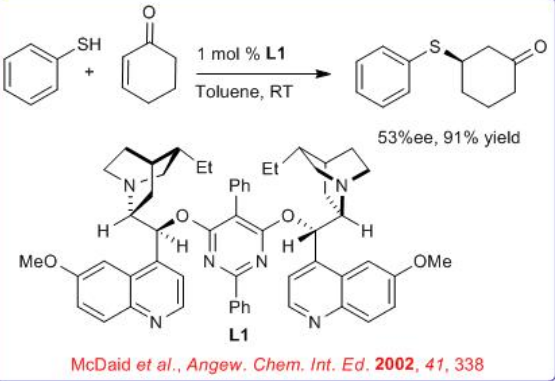
The catalytic asymmetric 1,4-addition of thiols to cyclic enones with modified cinchona alkaloid has been demonstrated (Scheme \(\PageIndex{3}\)). The Michael products can be isolated with high yield and enantioselectivity for a range of substances.
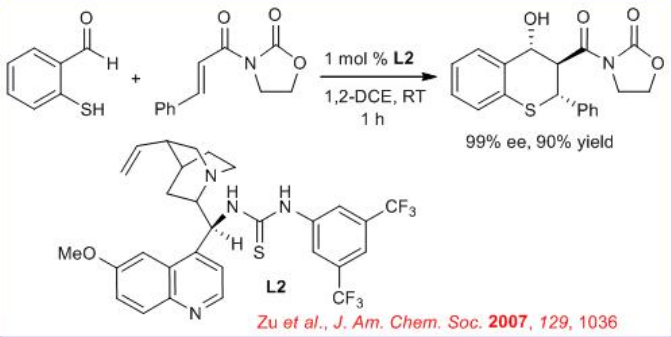
Later, tandem Michael-aldol reactions have been developed for the preparation of medicinally important chiral thiochromanes (Scheme \(\PageIndex{4}\)). This new one-pot process proceeds with 1 mol % of the cinchona alkaloid derived thiourea catalyst L2 , which synergistically activates both the Michael donor and acceptor.
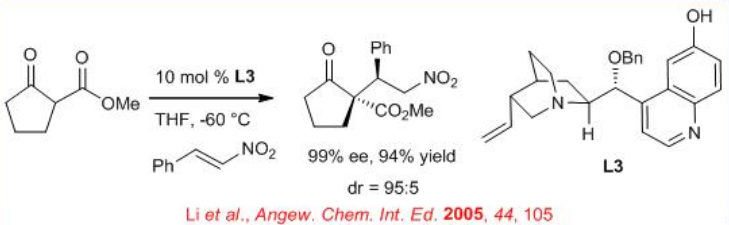
Similarly, the conjugate addition has been reported with catalyst L3 for a direct, stereocontrolled construction of adjacent carbon- or heteroatom-substituted quaternary and tertiary stereocenters from readily available starting β -ketoester (Scheme \(\PageIndex{5}\)).
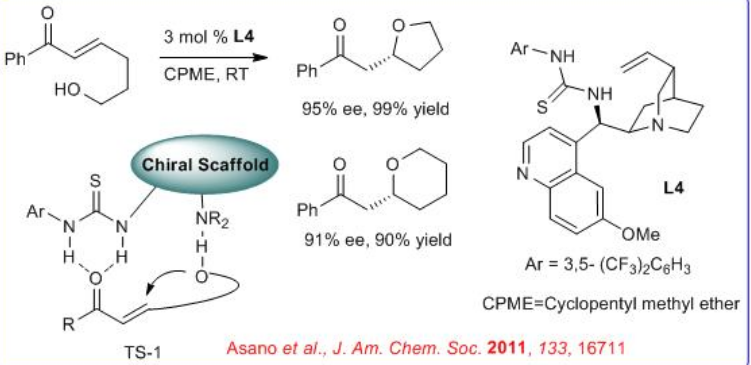
Chiral oxacyclic structures such as tetrahydrofuran rings are commonly found in many bioactive compounds. Cinchona-alkaloid-thiourea L4 catalyzes the cycloetherification of ε-hydroxy-α,β-unsaturated ketones with excellent enantioselectivity, even with low catalyst loadings at room temperature. The probable activation intermediate might go through TS-1.
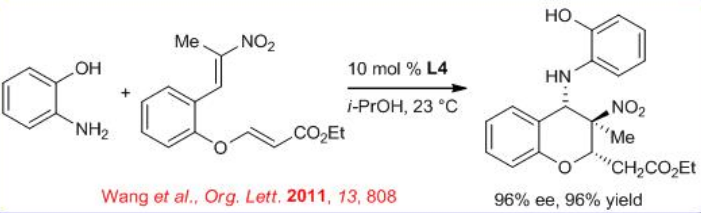
The catalyst L4 can also catalyze the domino aza-Michael–Michael reactions of anilines with nitroolefin enoates to afford chiral 4-aminobenzopyrans bearing two consecutive stereogenic centers and one quaternary stereocenter (Scheme \(\PageIndex{7}\)). The products can be isolated with high yield and enantioselectivity.
Chiral amine L5 has been used to activate α,β -unsaturated enones with nitro alkenes toward a well-defined enamine-iminium activation mode in presence of 2-fluorobenzoic acid as an additive. The reaction affords the Diels–Alder adduct bearing three or four stereogenic centers with high enantioselectivity (Scheme \(\PageIndex{8}\)). The extension of this process to other Michael acceptors such as N -benzyl maleimide leads to the formation of cyclohexanones with up to >99% ee
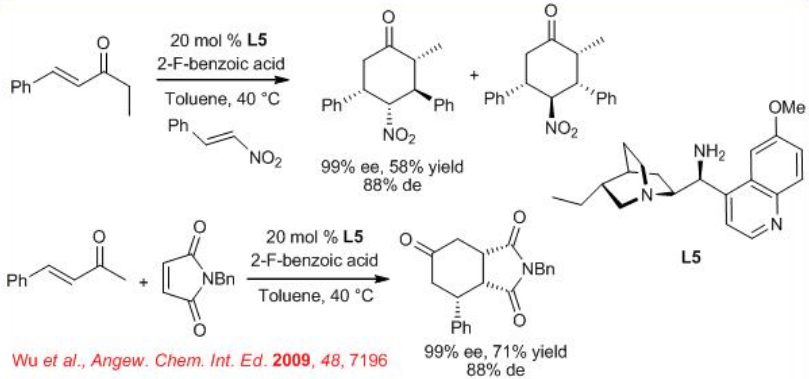
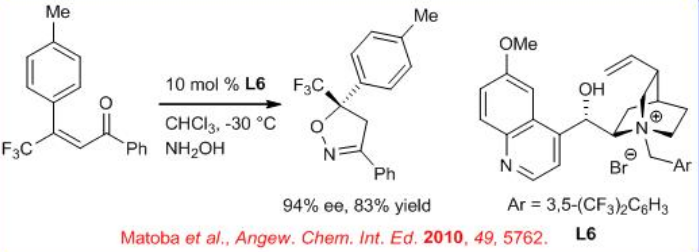
The synthesis of trifluoromethyl-substituted 2-isoxazolines can be accomplished by a domino Michael–cyclization–dehydration reaction of hydroxylamine (NH2OH) with a range of (E)- trifluoromethylated enone derivatives in the presence of N -3,5-bis(trifluoromethyl benzyl) quinidinium bromide L6 as a chiral phase transfer catalyst (Scheme \(\PageIndex{9}\)).
Aldol Reaction
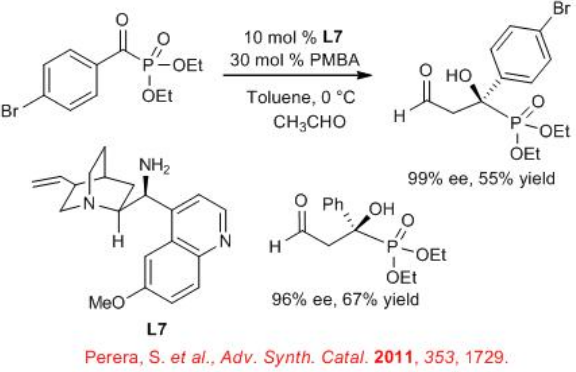
The cross-aldol reaction between enolizable aldehydes and α -ketophosphonates can be achieved using 9-amino-9-deoxy- epi -quinine L7 (Scheme 10). The reaction works especially well with acetaldehyde, which is a tough substrate for organocatalyzed cross-aldol reaction.
10.2.3 Henry Reaction
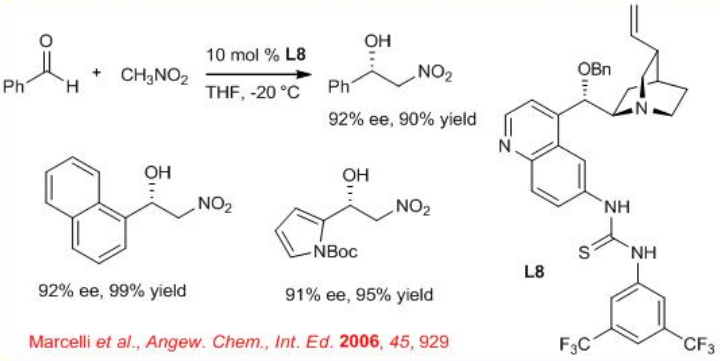
Henry reaction is a classical carbon-carbon bond forming reaction in organic synthesis. Aryl aldehydes react with nitromethane in the presence of 6'-thioureasubstituted cinchona alkaloid L8with high enantioselectivity (Scheme \(\PageIndex{11}\)). Hydrogen-bond donor at the C6′ of L8 has been found to induce preferential formation of one enantiomer.
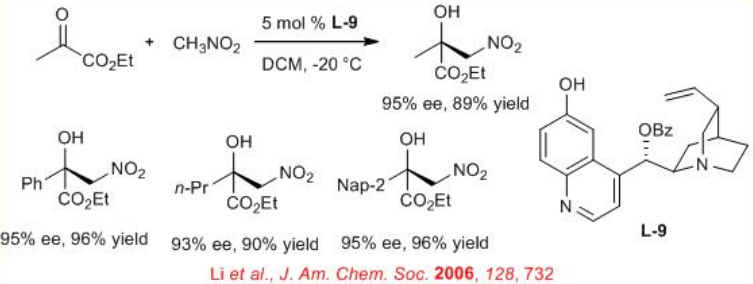
The 6'-OH cinchona alkaloid L-9 is an excellent catalyst for the reaction of α- ketoesters with nitromethane (Scheme \(\PageIndex{12}\)). The highly enantioenriched products from the Henry reaction could be elaborated to aziridines, β- lactams and α -alkylcysteines. This reaction is operationally simple and affords high enantioselectivity as well as good to excellent yield for a broad range of α-ketoesters .
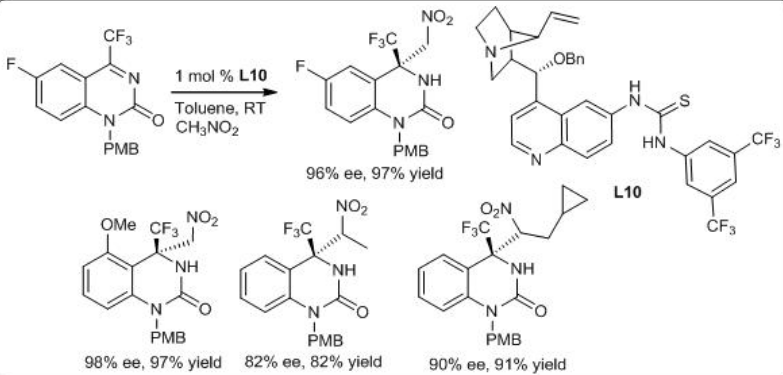
Bifunctional cinchona alkaloid-thiourea L10 can catalyze efficiently the aza-Henry reaction of cyclic trifluoromethyl ketimines with nitromethanes (Scheme \(\PageIndex{13}\)). The title reaction can provide biologically interesting chiral trifluoromethyl dihydroquinazolinone frameworks with high yield and enantioselectivity.
Hydroxyalkylation Reaction
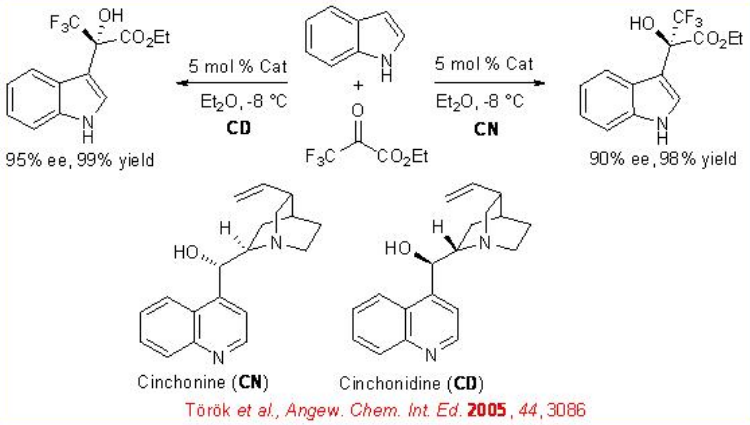
The readily available cinchonidine (CD) and cinchonine (CN) can be used for the catalysis of the hydroxyalkylation of heteroaromatics. For example, the hydroxyalkylation of indoles with ethyl-3,3,3-trifluropyruvate occurs to afford corresponding 3-substituted products in high yields and ee values (Scheme \(\PageIndex{14}\)) .