10.3: Thiourea Based Catalysis
- Page ID
- 168832
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Strecker Synthesis
In 1996 the first asymmetric organocatalytic Strecker synthesis appeared employing L1 as a catalyst (Scheme \(\PageIndex{1}\)). The reaction involves the addition of HCN to imines in the presence of diketopiperazine derivative with up to >99% ee.

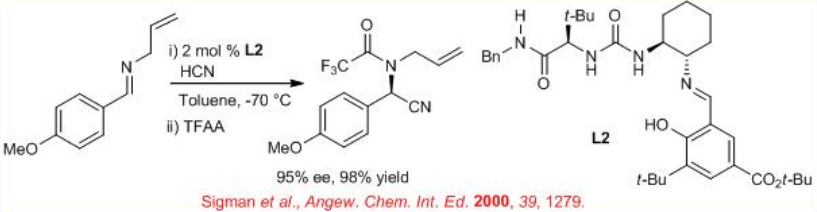
Subsequently, chiral thiourea derivative L2 has been used for this reaction to afford the cyanohydrins with 98% ee (Scheme \(\PageIndex{2}\)).
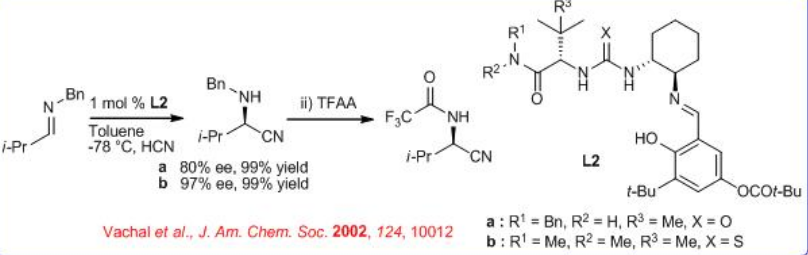
Further improvement in this reaction has been made employing thiourea derivative L3 (Scheme \(\PageIndex{3}\)). The active site of the catalyst, the relevant stereoisomer of the imine substrate and the solution structure of the imine−catalyst complex are elucidated using kinetics, structural activity and NMR experiments. An unusual bridging interaction between the imine and the urea hydrogens of the catalyst is identified.
Mannich Reaction
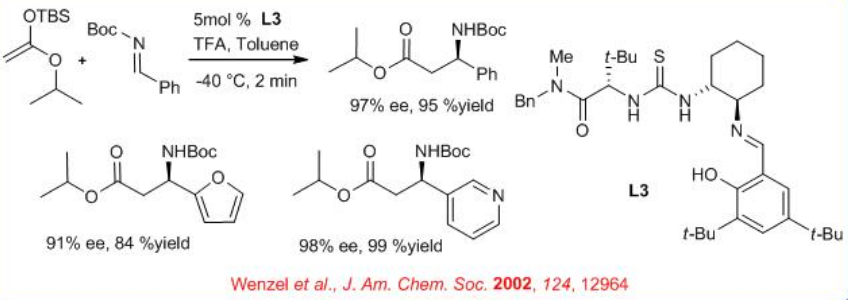
In parallel to Strecker reaction, the Mannich reaction of a wide variety of N -Boc aryl imines is studied in the presence of thiourea derivative L3 with high enantioselectivity (Scheme \(\PageIndex{4}\)). The catalyst L3 is as highly effective for the asymmetric addition of silyl ketene acetal derivatives to aldimines. From a steric and electronic standpoint, the N -Boc imine substrates utilized in this reaction are fundamentally different from the N -alkyl derivatives employed in the Strecker reaction.
Bifunctional thiourea derivative L4 can catalyze the Michael reaction of malonates with various nitro olefins in high enantioselectivity (Scheme \(\PageIndex{5}\)). The catalyst activates nucleophile by general base catalysis and electrophile by H-bonding to the nitro group. This methodology has been applied for enantioselective additions of substituted keto ester and double Michael additions of α,β -unsaturated ketoesters.
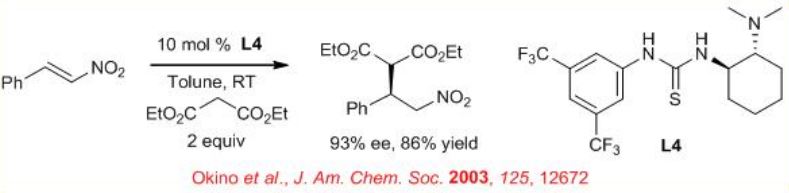
Chiral primary amine - thiourea L5 is effective for the direct conjugate addition of ketones to nitroalkenes (Scheme \(\PageIndex{6}\)) . The observed anti diastereoselectivity suggests the participation of a (Z) -enamine intermediate which is complementary to the diastereoselectivity obtained in analogous reactions involving (E) -enamines generated from secondary amine catalysts.
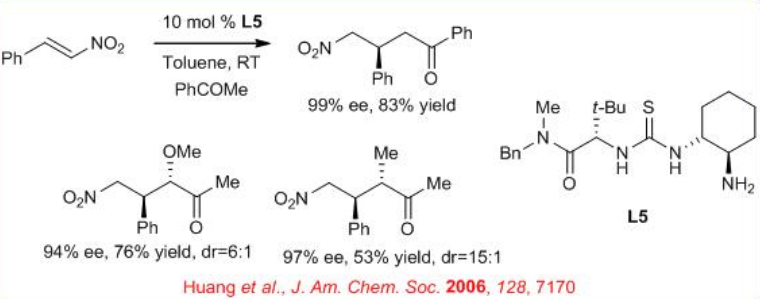
Likewise, the addition of a range of nitroalkanes to aromatic N -Boc imines has been shown using the thiourea derivative L6 with mostly anti diastereoselectivity (Scheme \(\PageIndex{7}\)).
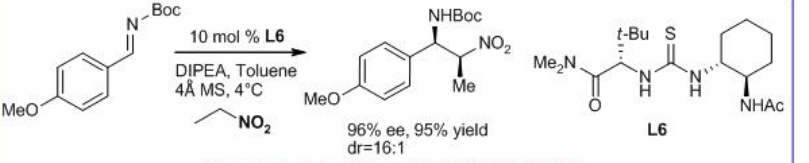
The thiourea catalyst L7 bearing 3,5-bis(trifluoromethyl) benzene and dimethylamino groups has been revealed to be efficient for the asymmetric Michael reaction of 1,3-dicarbonyl compounds to nitroolefins (Scheme \(\PageIndex{8}\)). This methodology has been applied for the total synthesis of (R)-(−)-baclofen. Reaction of 4-chloronitrostyrene and 1,3-dicarbonyl compound generates quaternary carbon center with 94% ee. Reduction of the nitro gruop to amine and subsequent cyclization, esterification and ring opening provides ( R )-(−)-baclofen in 38% yield.
The mechanism of above enantioselective Michael addition of acetyl acetone to a nitroolefin catalyzed by a thiourea-based chiral bifunctional organocatalyst has been investigated using density functional theory calculations and the results suggests that both substrates coordinate preferentially via bidentate hydrogen bonds (H-bond) (Scheme \(\PageIndex{9}\)). The deprotonation of the enol form of acetylacetone by the amine of the catalyst is found to occur easily, leading to an ion pair characterized by multiple H-bonds involving the thiourea unit as well. Two distinct reaction pathways have been explored toward the formation of the Michael product that differs in the mode of electrophile activation. Both reaction channels are shown to be consistent with the notion of non-covalent organocatalysis in that the transition states leading to the Michael adduct are stabilized by extensive H-bonded networks.
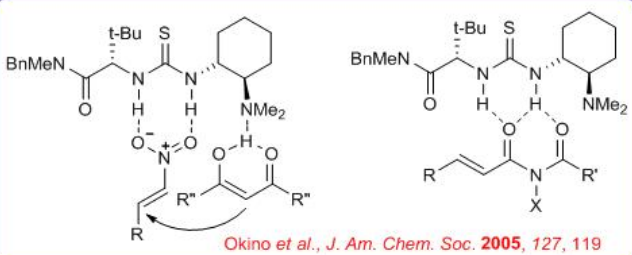
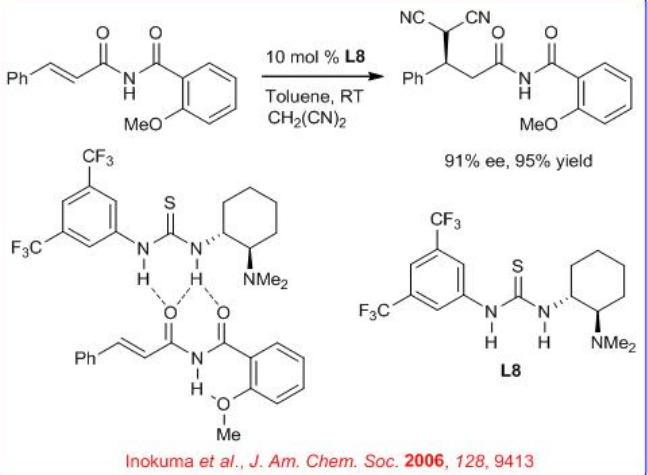
A thiourea-catalyzed asymmetric Michael addition of activated methylene compounds to α,β- unsaturated imides have been developed (Scheme \(\PageIndex{10}\)). N -Alkenoyl-2-methoxybenzamide is the best substrate among the corresponding benzamide derivatives bearing different substituents on the aromatic ring and react with several activated methylene compounds such as malononitrile, methyl α -cyanoacetate, and nitromethane with up to 93% ee. The reactivity can be attributed to the intramolecular H-bonding interaction between the N-H of the imide and the methoxy group of the benzamide moiety.
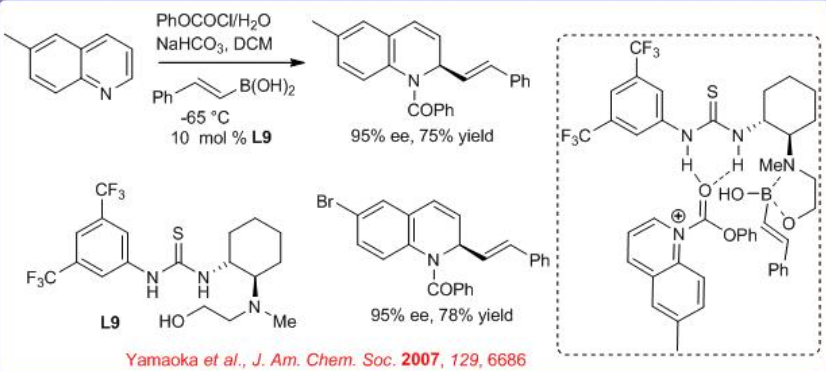
Thiourea catalyst L9 has been explored for the activation of quinoline with organoboronic acids to facilitate stereocontrol in the Petasis transformation even at low temperatures (Scheme \(\PageIndex{11}\)). The quinoline gets activated by formation of N-COBz with PhCOCl and a high degree of stereo control can be achieved using a combination of H2O and NaHCO3 as additives.
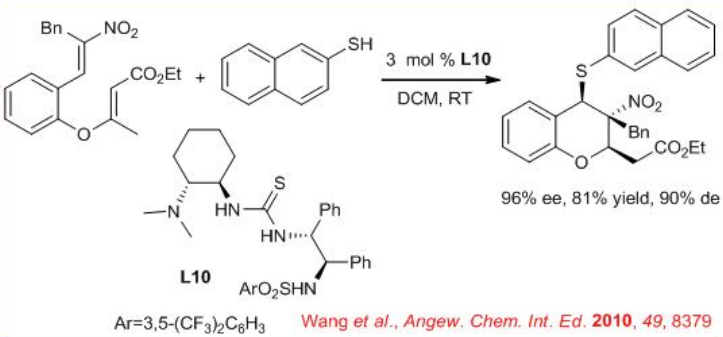
The domino thia-Michael–Michael reaction of thiols with nitro olefin enoates provides polyfunctionalized chroman derivatives in a highly stereoselective manner in the presence of thiourea L10 . Three consecutive stereogenic centers including one quaternary stereocenter can be generated with high enantioselectivity (Scheme \(\PageIndex{12}\)). The catalyst L10 activates nitroolefin enoates through H-bonding activation, and its tertiary amino moiety activates the nucleophilic thiols, forming an intermediate which undergo the intermolecular thia-Michael addition.
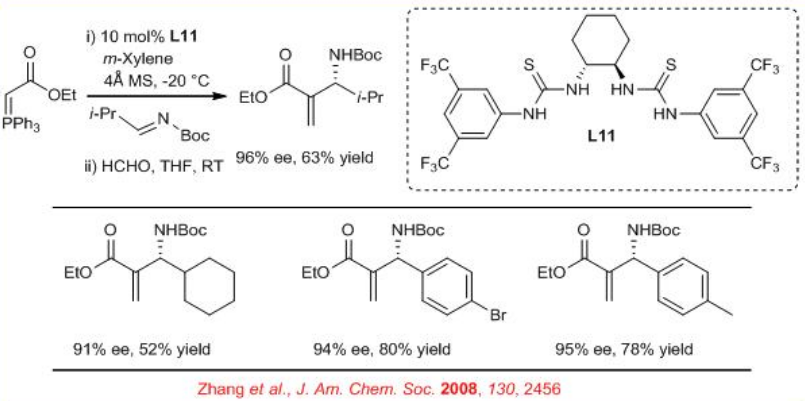
The synthesis of chiral N-Boc- β-Amino- α -methylene carboxylic esters can be performed by reaction of stabilized phosphorus ylides and Boc-protected aldimines in presence of readily available bisthiourea L11 (Scheme \(\PageIndex{13}\)). Subsequent reaction with formaldehyde provides a facile access to chiral N -Boc- β-amino- α -methylene carboxylic esters. The catalyst has been found to be recyclable.
Hydrophosphonylation Reactions
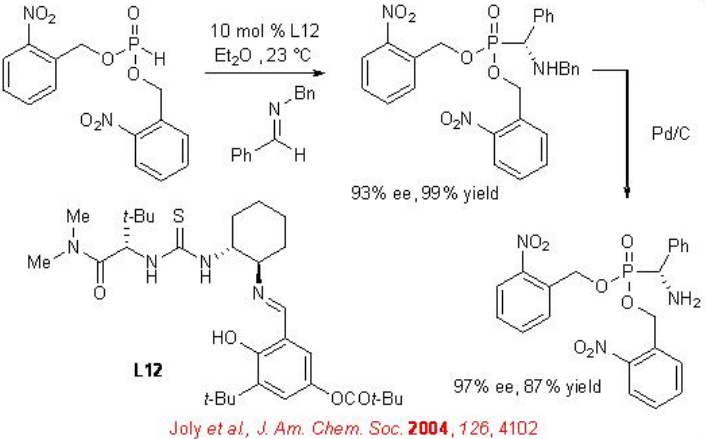
Chiral thiourea catalyst L12 has been used for highly enantioselective hydrophosphonylation of a wide range of N -benzyl imines (Scheme \(\PageIndex{14}\)). The hydrophosphonylated products can be readily deprotected by hydrogenolysis using Pd/C to provide chiral α-amino phosphonic acids with high enantioselectivity. This methodology provides general and convenient access for the synthesis of optically active α -amino phosphonates.