17.E: The Organic Chemistry of Vitamins (Exercises)
- Page ID
- 106404
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)P17.1: Here is a chance to test your ability to recognize reactions catalyzed by enzymes using three coenzymes - thiamine diphosphate, pyridoxal phosphate, and folate - that we have studied in this chapter. For each generalized reaction, look carefully at the transformation that is taking place, and decide which of the three coenzymes is likely to be required. Then, draw the single mechanistic step by which the bond identified by an arrow is broken or formed. In the cases where a double bond is indicated, show the mechanistic step in which the s bond is formed. In each case, your drawing should include the structure of the reactive part of the coenzyme, and should clearly show the role it plays in catalyzing the mechanistic step you are drawing.
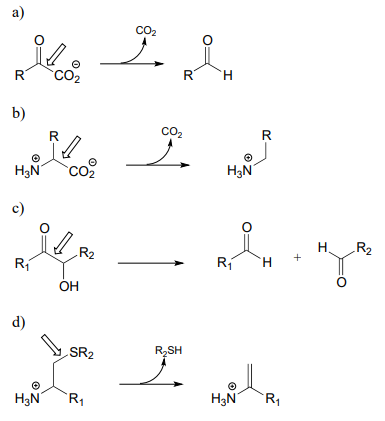

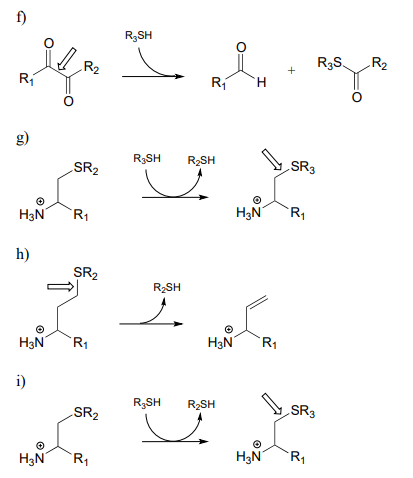
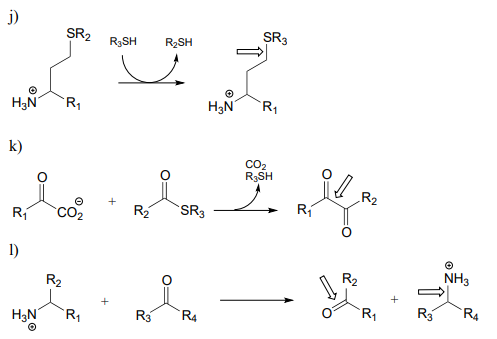

P17.2: The final step in the biosynthesis of the amino acid tryptophan is a PLP-dependent condensation between serine and indole, shown below (EC 4.2.1.20). The reaction mechanism involves steps that are familiar from this chapter, but also incorporates a reaction type we studied in chapter 14. Propose a mechanism.
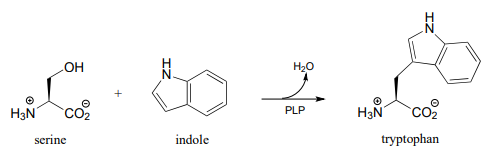
P17.3: Draw a reasonable mechanism for the following reaction, identifying the species denoted by questions marks. (Biochemistry 2012, 51, 3059)
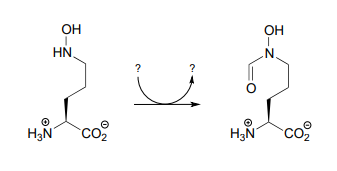
P17.4: Propose a mechanism for the reaction below, which is part of the anaerobic catabolism of alcohols in some species of bacteria. (ChemBioChem 2014, 15, 389)
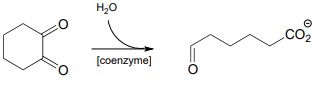
P17.5: Identify cosubstrate A and propose a mechanism for the reaction shown below, which was reported to occur in the thermophilic bacterium Thermosporothrix hazakensis. (ChemBioChem 2014 15, 527).
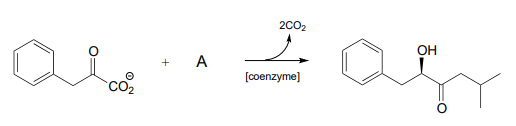
P17.6: Propose a mechanism for each of the reactions below, being sure to show the role played by the coenzyme (you need to determine which coenzyme is needed in each case).
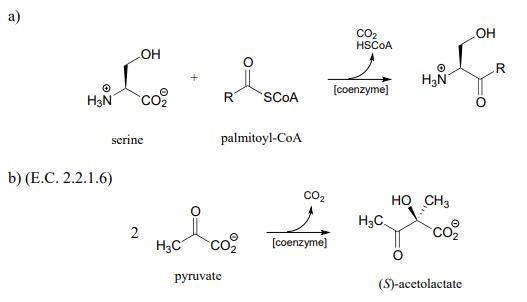
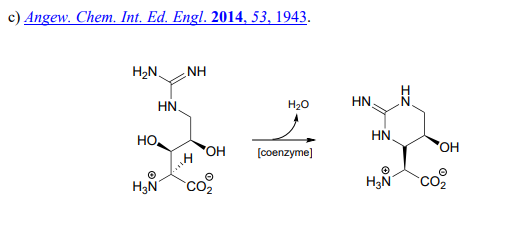
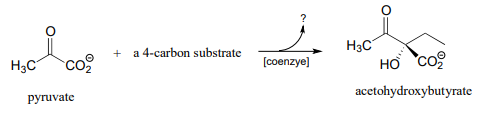
P17.7: Acetohydroxybutyrate is formed in a coenzyme-dependent reaction between pyruvate and a 4-carbon compound. What is a likely second substrate, coenzyme, and by-product (indicated below with a question mark)?
http://www.sciencedirect.com/science...67593105001043
P17.8: The 'benzoin condensation' reaction was discovered in the 19th century, and led eventually to a better understanding of \(ThDP\)-dependent reactions in the cell. In a traditional benzoin condensation reaction, cyanide ion (instead of ThDP) plays the role of electron acceptor. Enzyme-catalyzed benzoin condensation reactions are also known to occur in some bacteria: Pseudomonas fluorescens, for example, contains an enzyme that catalyzes the synthesis of (R)-benzoin.
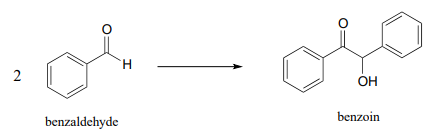
- Draw a mechanism for the enzyme-catalyzed (\(ThDP\)-dependent) benzoin condensation reaction.
- Draw a mechanism for the cyanide-catalyzed benzoin condensation reaction (non-enzymatic, basic conditions).
- The following \(ThDP\)-reaction was recently reported to be part of the biosynthetic pathway for clavulanic acid, a compound that inhibits the action of b-lactamases (b-lactamases are bacterial enzymes that hydrolyze penicillin-based antibiotic drugs, rendering them ineffective). As is typical for \(ThDP\)-dependent reactions, the first step is addition of the ylide form of the coenzyme to the substrate carbonyl. The next steps are (in order): dehydration, tautomerization, elimination of phosphate, conjugate addition of arginine, and finally hydrolytic cleavage of the coenzyme-product bond. Draw out a complete mechanism that corresponds to this description.
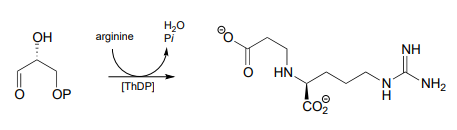
P17.9: Practice with PLP-dependent reactions:
- Propose a mechanism for this reaction, which is part of the tryptophan degradation pathway (EC 3.7.1.3).
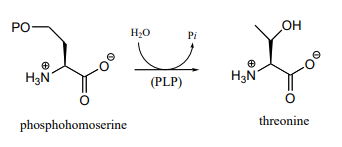
- Propose a mechanism for the final step of the threonine biosynthesis pathway (EC 4.2.3.1).
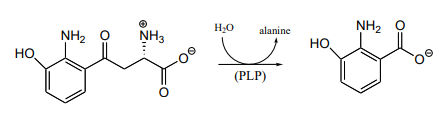
- Propose a mechanism for the reaction catalyzed by aspartate \(\beta \)-decarboxylase (EC 4.1.1.12), which converts aspartate to alanine in a PLP-dependent reaction.
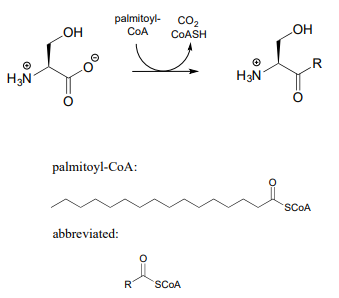
- Sphingolipids are a type of membrane lipid found in the membranes of all eukaryotic cells, and are most abundant in the cells of central the central nervous system. Synthesis of sphingolipids involves the PLP-dependent reaction below, catalyzed by serine palmitoyl transferase (EC 2.3.1.50). Propose a mechanism.
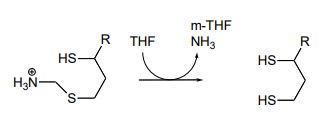
P17.10: As we saw in this chapter, PLP-dependent enzymes usually catalyze reactions involving amino acid substrates. Here is an exception, a PLP-dependent \(\beta \)-elimination reaction in the folate biosynthetic pathway (EC 4.1.3.38). Propose a mechanism for this reaction.
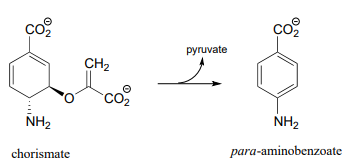
P17.11: The final step in the degradation pathway for the amino acid glycine (also known as the 'glycine cleavage system') is shown below. Propose a likely mechanism, given that evidence suggests that \(CH_2NH_2^+\) is an intermediate.
P17.12: As we saw in chapter 15, the usual biochemical role of \(NAD^+\) is to act as a hydride acceptor in dehydrogenation reactions. An exception is the reaction catalyzed by the histidine degradation pathway enzyme urocanase (EC 4.2.1.49).
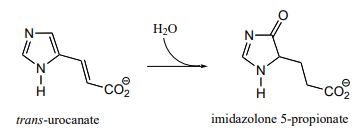
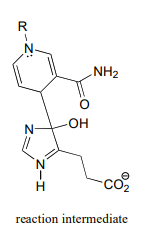
In this reaction, \(NAD^+\) acts as a catalytic, electron-sink coenzyme - it temporarily accepts electrons from a pi bond in the substrate, resulting in a covalent substrate-\(NAD\) adduct. This allows a key isomerization step to occur on the substrate through a protonation-deprotonation mechanism, followed by addition of water, cleavage of the substrate-\(NAD\) adduct to regenerate \(NAD^+\), and finally tautomerization to the product. Propose a mechanism that fits this description, and involves the intermediate below.
Pathway prediction problems
P17.13: Propose a multistep pathway for each of the following transformations. All involve at least one step requiring PLP, \(ThDP\), or folate.
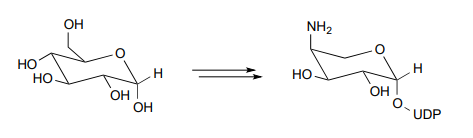
- Below is portion of the biosynthesis of a modified membrane lipid in Salmonella and other pathogenic bacteria. The modified membrane confers antibiotic resistance to the bacterium. Biochemistry 2014, 53, 796
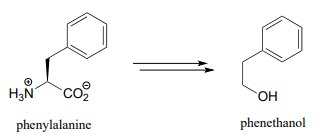
- Below is the biosynthetic pathway for phenethanol in yeast. Phenethanol, which has a rose scent, is commonly used as a fragrance - this pathway has been proposed as a potential 'green' enzymatic synthesis to replace the traditional industrial synthesis, which uses toxic reagents.
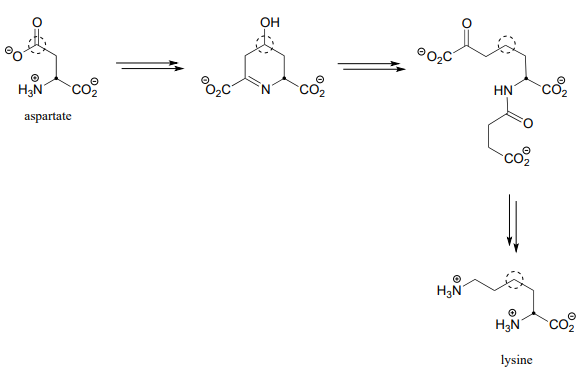
- Below is an incomplete pathway diagram for the biosynthesis of the amino acid lysine, starting from aspartate. Fill in the missing steps and reactants/coenzymes to complete the diagram. The solid dot and dashed circle are provided to help you to trace two of the carbons from substrate to product.

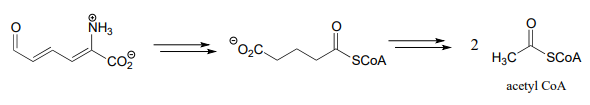
- Below is the second half of the tryptophan degradation pathway. Fill in the missing steps and reactants/coenzymes to complete the diagram.
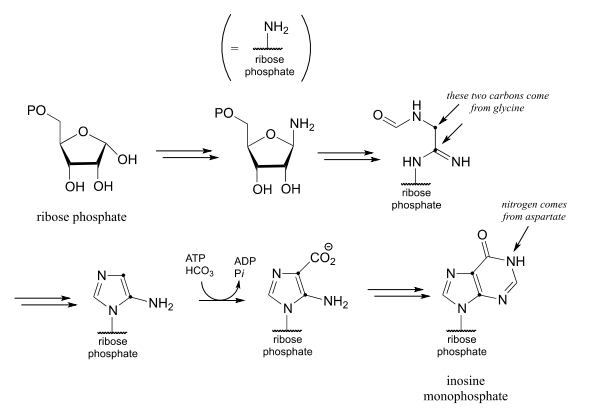
- Below is an incomplete pathway diagram for the biosynthesis of inosine monophosphate, a precursor to the nucleotides adenosine monophosphate (\(AMP\)) and guanosine monophosphate (\(GMP\)). Fill in missing steps and reactants/coenzymes to complete the diagram. Note that one enzymatic step is provided (this is a carboxylation reaction of a type that we have not studied).
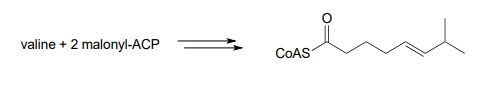
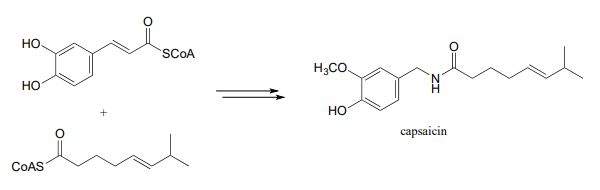
- We begin our study of organic chemistry with a story about a hot pepper eating contest in Wisconsin (see the introduction the Chapter 1), and a compound called capsaicin which causes the 'hot' in hot peppers. As our last problem, let's try to predict some of the key steps in the biosynthesis of capsaicin.
Phase 1:
Phase 2:
Contributors and Attributions
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)


