17.5: Folate
- Page ID
- 106403
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Folate, or vitamin \(B_9\), is essential for a variety of important reactions in nucleotide and amino acid metabolism. The reactive part of folate is the pterin ring system, shown in red below. The conventional atom numbering system for folate is also indicated.
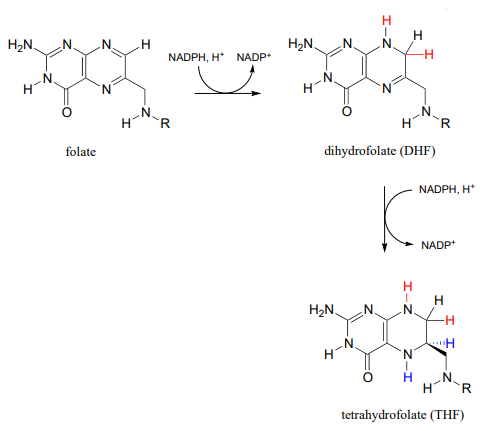
Active forms of folate
Folate is active as a coenzyme in its reduced forms, dihydrofolate and tetrahydrofolate, which are formed by \(NADPH-\)dependent imine hydrogenation steps (section 15.3).
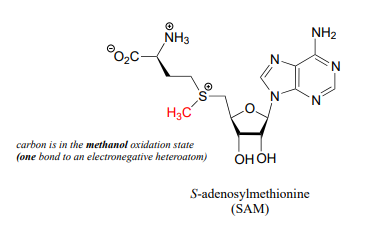
The metabolic role of folate is to serve as a donor or acceptor in single-carbon transfer reactions. How is folate different from \(S\)-adenosylmethionine (\(SAM\), section 8.8) which also serves as a single-carbon donor? Recall that \(SAM\) donates a single carbon in the form of a methyl group: essentially, the single carbon of \(SAM\) is in the methanol (\(CH_3OH\)) oxidation state, because it has only one bond to a heteroatom (specifically, to sulfur). (Refer to section 15.1 for a review of oxidation states).
Folate coenzymes, on the other hand, can carry a single carbon in the formaldehyde and formate oxidation states, in addition to the methanol oxidation state. By 'formaldehyde' and 'formate' oxidation state, we mean that the carbon has two and three bonds to heteroatoms, respectively.
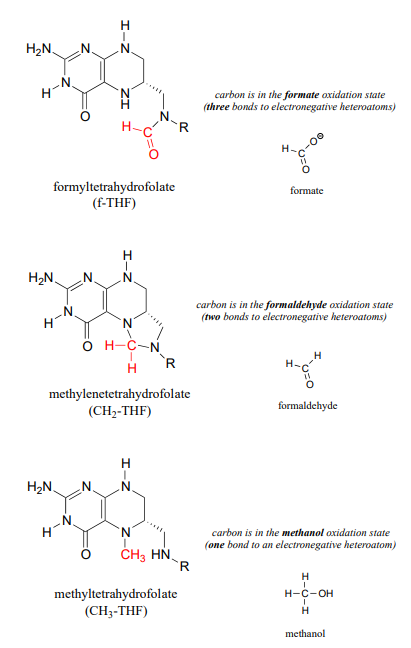
Some key reactions in nucleic acid and amino acid metabolic pathways involve transfer of a single carbon in the formaldehyde or formate states. However, this could present problems. Formaldehyde by itself is very toxic: in particular, it tends to spontaneously form unwanted crosslinks between amine groups (eg. lysine side chains) in proteins.
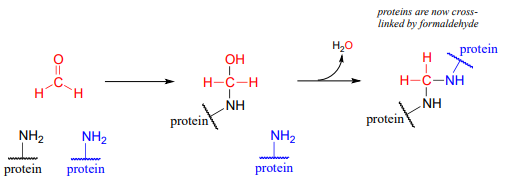
fig 53
Free formaldehyde is too reactive, and would cause damage to a cell. The \(CH_2-THF\) coenzyme is stable in solution, but in the active site of certain enzymes it is reactive enough to serve as a formaldehyde donor, as we will see shortly.
Free formate, on the other hand, is a carboxylate, and we know from chapter 11 that carboxylates are not reactive in acyl substitution steps. Formate could be activated by phosphorylation, of course, but the resulting formyl phosphate would be too reactive in many enzyme active sites. A 'happy medium' has been found in which carbons in the formate oxidation state are carried by folate in the form of \(f-THF\): once again, the carbon donor is stable in solution, but sufficiently reactive in certain enzyme active sites to accomplish controlled transfer of a formate group.
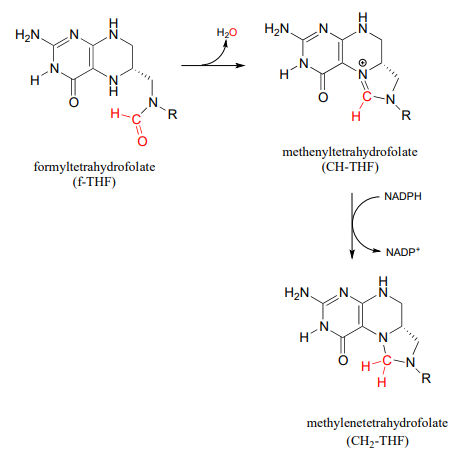
Formation of formyl-\(THF\) and methylene-\(THF\)
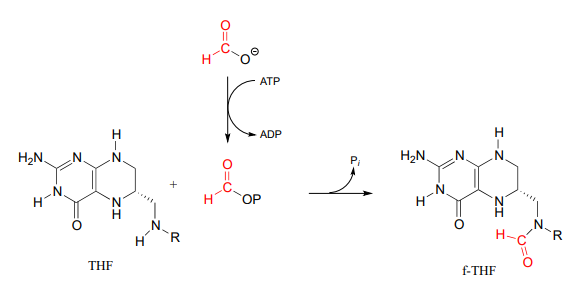
Formyltetrahydrofolate (\(f-THF\)) is formed from \(THF\) and a formate molecule which has been activated by phosphorylation (formyl phosphate, as stated in the paragraph above, is a high reactive intermediate, but is held inside the enzyme's active site for immediate reaction with the incoming amine group of \(THF\)).
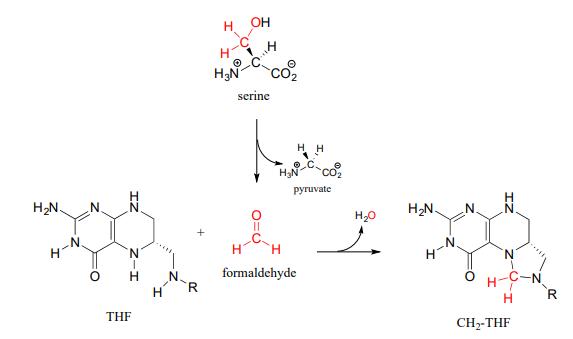
There are two main metabolic routes to \(CH_2-THF\). One route is just the last step of the serine hydroxymethyltransferase reaction we have already seen in section 17.1: the formaldehyde formed in the PLP-dependent phase of the reaction stays in the active site, and the oxygen is displaced by successive attacks from the amine nucleophiles at the 5 and 10 positions of \(THF\). (Notice the similarity to the formaldehyde-protein crosslinking reaction shown earlier in this section.)
In the second route, \(f-THF\) is dehydrated, then the resulting methenyl-\(THF\) intermediate is reduced by \(NADPH\).
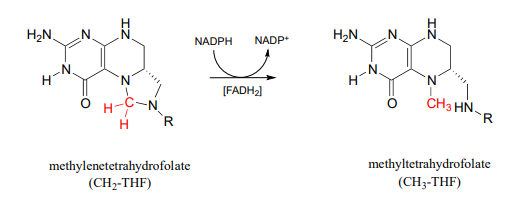
Methylene-\(THF\) (\(CH_2-THF\)) is reduced to methyl-\(THF\) (\(CH_3-THF\)) in a flavin-dependent reaction. Biochemistry 2001, 40, 6216
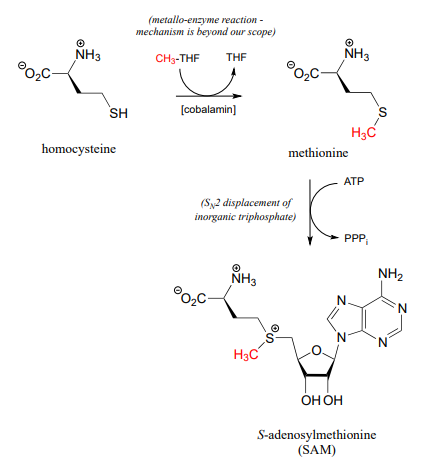
Recall from the introduction to this chapter that babies who were in the womb during the Dutch Hunger Winter faced a higher risk, when they reached adulthood, of conditions such as obesity and schizophrenia, most likely caused by disruptions in the \(S\)-adenosylmethionine-dependent methylation of their DNA, which was in turn caused by their mothers not getting enough folate. \(CH_3-THF\) is the source of the methyl group in methionine, which ultimately becomes the methyl group in \(SAM\). The methylation of homocysteine to methionine (below) involves a cobalt-containing coenzyme called cobalamin, but the mechanism for this reaction is beyond the scope of our discussion. The second reaction below (formation of \(SAM\)) is simply an \(SN_2\) displacement of the inorganic triphosphate leaving group on ATP by the nucleophilic sulfur in methionine.
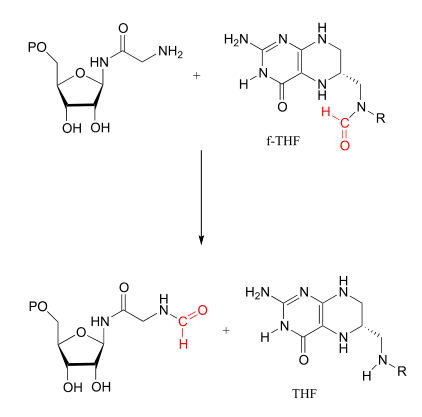
Single-carbon transfer with formyl-\(THF\)
There are two important \(f-THF\)-dependent formylation steps in the biosynthetic pathways for purine nucleophiles. Both are simply transamidation reactions: in other words, conversion by the nucleophilic acyl substitution mechanism (chapter 11) of one amide to another.
Glycinamide ribonucleotide transformylase reaction:
Aminoimidazole carboxamide transformylase reaction:
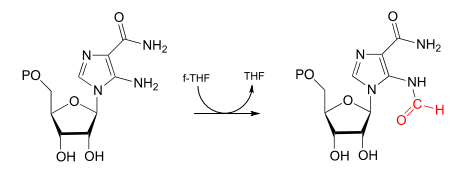
Which of the two transformylase reactions above would you expect to be more kinetically favorable? Explain your reasoning.
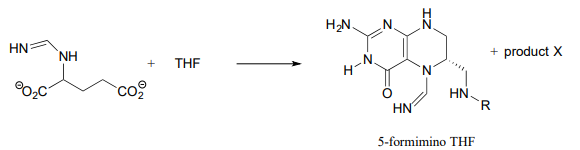
Predict the structure of 'product X' in the reaction below, from the histidine degradation pathway.
Single-carbon transfer with methylene-\(THF\)
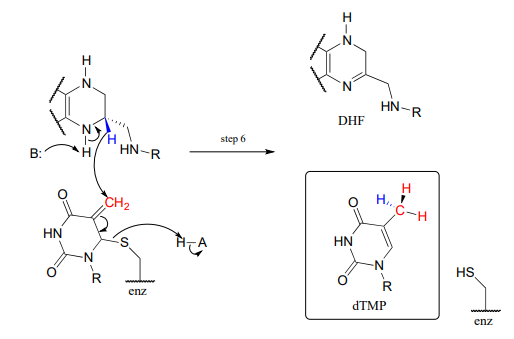
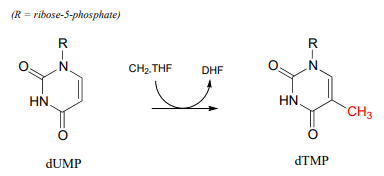
\(CH_2-THF\) serves as a single-carbon donor in a somewhat complicated reaction in the biosynthesis of the DNA monomer doexythymidine monophosphate (\(dTMP\)).
The reactions begins with the five-membered ring of \(CH_2-THF\) breaking apart to create an imine intermediate (step 1 below). The imine becomes an electrophile in a conjugate addition step (section 13.4, steps 2-3 below). Note that after step 1, the second ring in the pterin system of the coenzyme is abbreviated for clarity.
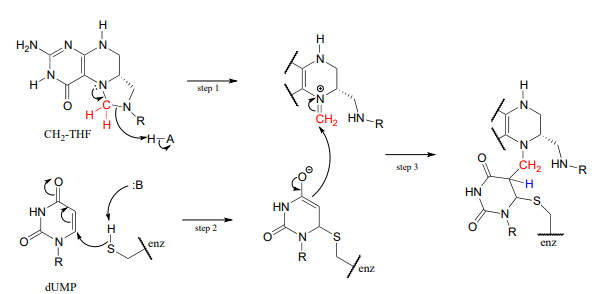
Next, tetrahydrofolate is eliminated in an \(E1cb\) elimination mechanism (steps 4 and 5). Notice that this is where the single carbon is transferred from methyl-\(THF\) to dUMP.
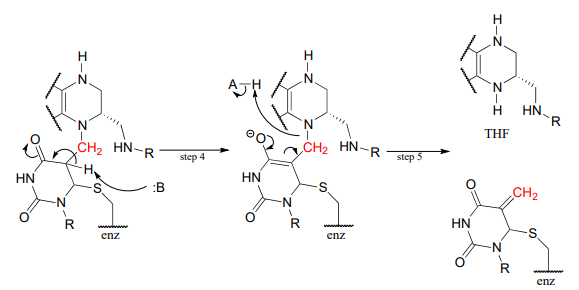
The final step in the mechanism is where it gets really interesting: a hydride ion is transferred from the tetrahydrofolate coenzyme to the methylene (\(CH_2\)) group on the deoxynucleotide substrate. Essentially, this is a conjugated \(SN_2\) step with hydride as the nucleophile and the active site cysteine as the leaving group.