20.8: Polysaccharides
- Page ID
- 22307
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Cellulose
The fibrous tissue in the cell walls of plants contains the polysaccharide cellulose, which consists of long chains of glucose units, each of which is connected by a \(\beta\)-glucoside link to the \(\ce{C_4}\) hydroxyl of another glucose as in the disaccharide cellobiose (i.e., \(\beta\)-1,4):
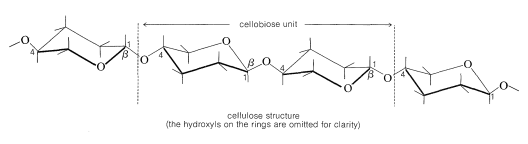
Indeed, enzymatic hydrolysis of cellulose leads to cellobiose. The molecular weight of cellulose varies with the source but is usually high. Cotton cellulose appears to have about 3000 glucose units per molecule.
The natural fibers obtained from cotton, wood, flax, hemp, and jute all are cellulose fibers and serve as raw materials for the textile and paper industries. In addition to its use as a natural fiber and in those industries that depend on wood as a construction material, cellulose is used to make cellulose acetate (for making rayon acetate yarn, photographic film, and cellulose acetate butyrate plastics), nitric acid esters (gun cotton and celluloid\(^7\)), and cellulose xanthate (for making viscose rayon fibers). The process by which viscose rayon is manufactured involves converting wood pulp or cotton linters into cellulose xanthate by reaction with carbon disulfide and sodium hydroxide:
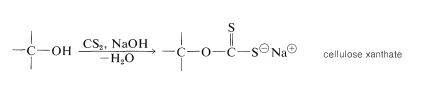
The length of the chains of the cellulose decreases about 300 monomer units in the process. At this point, the cellulose is regenerated in the form of fine filaments by forcing the xanthate solution through a spinneret into an acid bath:
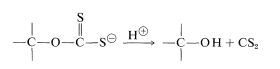
A few animals (especially ruminants and termites) are able to metabolize cellulose, but even these animals depend on appropriate microorganisms in their intestinal tracts to hydrolyze the \(\beta\)-1,4 links; other animals, including man, cannot utilize cellulose as food because they lack the necessary hydrolytic enzymes. However, such enzymes are distributed widely in nature. In fact, deterioration of cellulose materials - textiles, paper, and wood - by enzymatic degradation (such as by dry rot) is an economic problem that is not yet adequately solved. Efforts to turn this to advantage through enzymatic hydrolysis of cellulose to glucose for practical food production have not been very successful (Section 25-12).
Starch and Related Compounds
A second, very widely distributed polysaccharide is starch, which is stored in the seeds, roots, and fibers of plants as a food reserve - a potential source of glucose. The chemical composition of starch varies with the source, but in any one starch there are two structurally different polysaccharides. Both consist entirely of glucose units, but one is a linear structure (amylose) and the other is a branched structure (amylopectin).
The amylose form of starch consists of repeating 1,4-glucopyranose links as in cellulose, but unlike cellulose the linkage is \(\alpha\) rather than \(\beta\) (i.e., \(\alpha\)-1,4):
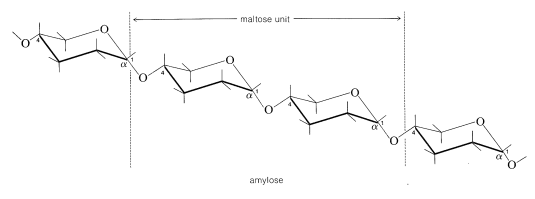
Hydrolysis by the enzyme diastase leads to maltose.
In amylopectin, amylose chains are joined by \(\alpha\)-1,6 linkages:
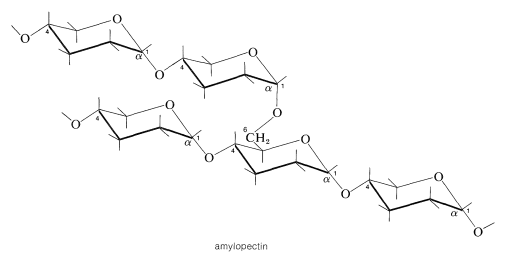
Animals also store glucose in the form of starchlike substances called glycogens. These substances resemble amylopectin more than amylose in that they are branched chains of glucose units with \(\alpha\)-1,4- and \(\alpha\)-1,6-glucoside links.
Starch is used in paper manufacture and in the textile and food industries. Fermentation of grain starches is an important source of ethanol. Hydrolysis of starch catalyzed by hydrochloric acid results in a syrupy mixture of glucose, maltose, and higher-molecular-weight saccharides. This mixture is called dextrin and is marketed as corn syrup. The hydrolysis does not proceed all the way to glucose because the \(\alpha\)-1,6 glucosidic link at the branch point is not easily hydrolyzed. Enzymes also catalyze hydrolysis of starch, but the enzyme \(\alpha\) amylase is specific for \(\alpha\)-1,4 links and, like acid-catalyzed hydrolysis, gives a mixture of glucose, maltose, and polysaccharides (dextrin). The enzyme \(\alpha\)-1,6-glucosidase can hydrolyze the \(\alpha\)-1,6 links at the branch points and, when used in conjunction with \(\alpha\) amylase, completes the hydrolysis of starch to glucose.
A very interesting group of polysaccharides isolated from cornstarch hydrolysates are known as cyclodextrins. One of these compounds, cyclohexaamylose, is a large doughnut-shaped molecule with a central cavity that literally can engulf a small, relatively nonpolar organic molecule and hold it in water solution, similar to a micelle (Section 18-2F). As with micelles, unusual reactivity is exhibited by the bound molecules. An example is the change in the ortho-para ratio in electrophilic substitution of methoxybenzene by hypochlorous acid, \(\ce{HOCl}\), in the presence and absence of cyclohexaamylose:
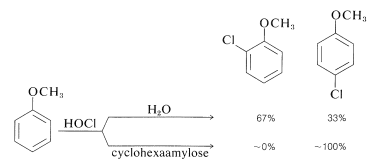
Apparently the cyclohexaamylose wraps around the methoxybenzene in such a way as to protect the ortho carbons from attack by \(\ce{HOCl}\) but to leave the para carbon exposed. It is this kind of specificity that we need to generate in reactions before we can claim to have synthetic reactions under control.
Other Important Polysaccharides
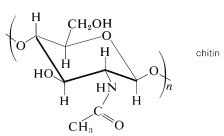
Many polysaccharides besides starch and cellulose are important components of animal tissues, or play a vital role in biochemical processes. One example is chitin, a celluloselike material that is the structural component of the hard shells of insects and crustaceans. The difference between chitin and cellulose is that instead of being a polymer of glucose, chitin is a polymer of 2-deoxy-2-\(\ce{N}\)-ethanamidoglucose (\(\ce{N}\)-acetyl-\(\beta\)-\(D\)-glucosamine):
Heparin is a very important and complex polysaccharide derivative that occurs in intestinal walls and has a major use as a blood anticoagulant, especially in connection with artificial kidney therapy. Heparin also has shown great promise in the treatment of patients with extensive burns, by promoting blood circulation to burn-damaged tissue. The structure of heparin can not be defined precisely because its composition depends on the source of supply. The major components of the polysaccharide chain are \(D\)-glucuronic acid, \(L\)-iduronic acid, and the same 2-deoxy-2-aminoglucose (\(D\)-glucosamine) that is a constituent of chitin (although in heparin it occurs as the \(\alpha\) anomer):
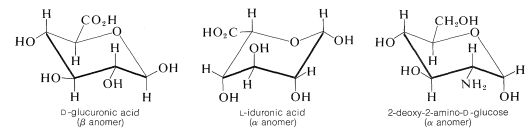
The general construction of heparin involves the linkage of the anomeric carbons of one of the components with the 4-hydroxyl of another. A key feature of the heparin structure is the presence of sulfate groups that occur as hydrogen sulfate esters (Section 15-5B) and as sulfamido groups, \(\ce{-NHSO_3H}\), on the 2-deoxy-2-amino-\(D\)-glucose units in the chain. Hydrogen sulfate groups also are located on the 2-hydroxyls of the \(L\)-iduronic acid units of the chain. In addition there are \(\ce{N}\)-ethanoyl groups attached to some of the 2-deoxy-2-amino-\(D\)-glucose nitrogens that are not connected to \(\ce{-SO_3H}\).
Heparin is clearly an extraordinarily complex substance with many highly polar groups, and its mode of action as an anticoagulant is not clear. At present, because of increases in the use of artificial kidney machines, heparin is in rather short supply.
Among the plant polysaccharides are the pectins, which are used as jelling agents in the making of preserves and jellies from fruit. Also important are the alginates from seaweeds and gums from trees, which are used as stabilizers and emulsifiers in the food, pharmaceutical, cosmetic, and textile industries. The pectins principally are polysaccharides of the methyl ester of \(D\)-galacturonic acid, whereas the alginates are polysaccharides made up of varying proportions of \(D\)-mannuronic acid and \(L\)-guluronic acid. The plant gums are similar materials.
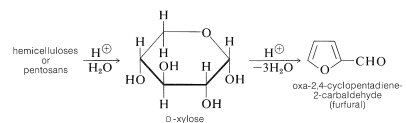
There are other polysaccharides besides cellulose in the cell walls of plants. These are called hemicelluloses, but the name is misleading because they are unrelated to cellulose. Those that are made of pentose sugars (mainly xylose) are most abundant. They accumulate as wastes in the processing of agricultural products, and on treatment with acids that yield a compound of considerable commercial importance, oxa-2,4-cyclopentadiene-2-carbaldehyde (furfural):
\(^7\)Celluloid, one of the first plastics, is partially nitrated cellulose (known as pyroxylin) plasticized with camphor.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


