23.7: Batteries
- Page ID
- 53974
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Alessandro Volta developed the first "voltaic cell" in 1800. This battery consisted of alternating disks of zinc and silver, with pieces of cardboard soaked in brine that separated the disks. There were no voltmeters at the time (and no insight that the electric current was due to electron flow). So, Volta had to rely on another measure of battery strength: the amount of shock produced (it's never a good idea to test things on yourself). He found that the intensity of the shock increased with the number of metal plates in the system. Devices with twenty plates produced a shock that was quite painful. It's lucky that we have voltmeters today to measure electric current, instead of having to test the voltage out for ourselves! Two variations on the basic voltaic cell are the dry cell and the lead storage battery.
Batteries: Dry Cells
Many common batteries, such as those used in a flashlight or remote control, are voltaic dry cells. These batteries are called dry cells because the electrolyte is a paste. They are relatively inexpensive, but do not last a long time and are not rechargeable.
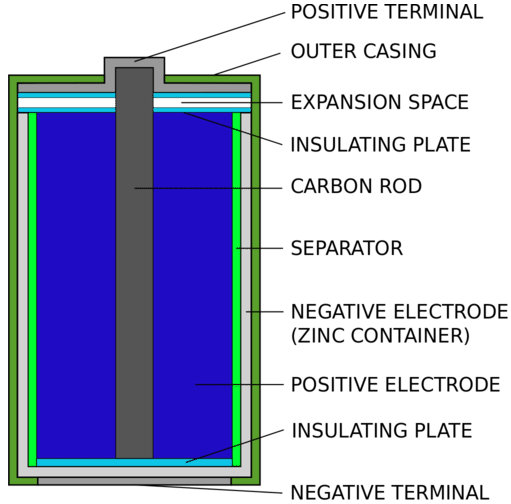
In the zinc-carbon dry cell, the anode is a zinc container, while the cathode is a carbon rod through the center of the cell. The paste is made of manganese (IV) oxide \(\left( \ce{MnO_2} \right)\), ammonium chloride \(\left( \ce{NH_4Cl} \right)\), and zinc chloride \(\left( \ce{ZnCl_2} \right)\) in water. The half-reactions for this dry cell are:
Anode (oxidation):
\[\ce{Zn} \left( s \right) \rightarrow \ce{Zn^{2+}} \left( aq \right) + 2 \ce{e^-}\nonumber \]
Cathode (reduction):
\[2 \ce{MnO_2} \left( s \right) + 2 \ce{NH_4^+} \left( aq \right) + 2 \ce{e^-} \rightarrow \ce{Mn_2O_3} \left( s \right) + 2 \ce{NH_3} \left( aq \right) + \ce{H_2O} \left( l \right)\nonumber \]
The paste prevents the contents of the dry cell from freely mixing, so a salt bridge is not needed. The carbon rod is a conductor only and does not undergo reduction. The voltage produced by a fresh dry cell is \(1.5 \: \text{V}\), but decreases during use.
An alkaline battery is a variation on the zinc-carbon dry cell. The alkaline battery has no carbon rod and uses a paste of zinc metal and potassium hydroxide, instead of a solid metal anode. The cathode half-reaction is the same, but the anode half-reaction is different.
Anode (oxidation):
\[\ce{Zn} \left( s \right) + 2 \ce{OH^-} \left( aq \right) \rightarrow \ce{Zn(OH)_2} \left( s \right) + 2 \ce{e^-}\nonumber \]
Advantages of the alkaline battery are that it has a longer shelf life, and the voltage does not decrease during use.
Lead Storage Batteries
A battery is a group of electrochemical cells combined together as a source of direct electric current at a constant voltage. Dry cells are not true batteries since they are only one cell. The lead storage battery is commonly used as the power source in cars and other vehicles. It consists of six identical cells joined together, each of which has a lead anode and a cathode made of lead (IV) oxide \(\left( \ce{PbO_2} \right)\) packed on a metal plate.
The cathode and anode are both immersed in an aqueous solution of sulfuric acid, which acts as the electrolyte. The cell reactions are:
Anode (oxidation):
\[\ce{Pb} \left( s \right) + \ce{SO_4^{2-}} \left( aq \right) \rightarrow \ce{PbSO_4} \left( s \right) + 2 \ce{e^-}\nonumber \]
Cathode (reduction):
\[\ce{PbO_2} \left( s \right) + 4 \ce{H^+} \left( aq \right) + \ce{SO_4^{2-}} \left( aq \right) + 2 \ce{e^-} \rightarrow \ce{PbSO_4} \left( s \right) + 2 \ce{H_2O} \left( l \right)\nonumber \]
Overall:
\[\ce{Pb} \left( s \right) + \ce{PbO_2} \left( s \right) + 4 \ce{H^+} \left( aq \right) + 2 \ce{SO_4^{2-}} \left( aq \right) \rightarrow 2 \ce{PbSO_4} \left( s \right) + 2 \ce{H_2O} \left( l \right)\nonumber \]
Each cell in a lead storage battery produces \(2 \: \text{V}\), so a total of \(12 \: \text{V}\) is generated by the entire battery. This is used to start a car or power other electrical systems.
Unlike a dry cell, the lead storage battery is rechargeable. Note that the forward redox reaction generates solid lead (II) sulfate which slowly builds up on the plates. Additionally, the concentration of sulfuric acid decreases. When the car is running normally, its generator recharges the battery by forcing the above reactions to run in the opposite, or nonspontaneous, direction.
\[2 \ce{PbSO_4} \left( s \right) + 2 \ce{H_2O} \left( l \right) \rightarrow \ce{Pb} \left( s \right) + \ce{PbO_2} \left( s \right) + 4 \ce{H+} \left( aq \right) + 2 \ce{SO_4^{2-}} \left( aq \right)\nonumber \]
This reaction regenerates the lead, lead (IV) oxide, and sulfuric acid needed for the battery to function properly. Theoretically, a lead storage battery should last forever. In practice, the recharge is not \(100\%\) efficient, because some of the lead (II) sulfate falls from the electrodes and collects on the bottom of the cells.
Summary
- Construction of a dry cell and a battery are given.
- Chemical reactions for both types are described.

