4.6: Energetics of chemical reactions
- Page ID
- 372946
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Energetics deals with the energies involved in chemical reactions. There are two types of energies: the first is related to thermodynamics which is the energy released or absorbed when the reactants convert to the products, and the second is related to the kinetics of the reaction, i.e., the energy that the reacting molecules must possess to surpass the energy barrier for conversion to the products.
Reaction coordinate diagram
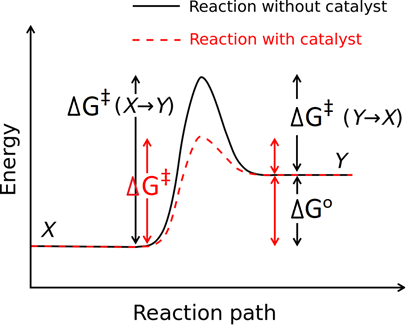
The progress of the reaction versus energy graph is shown in Fig. 4.7.1.
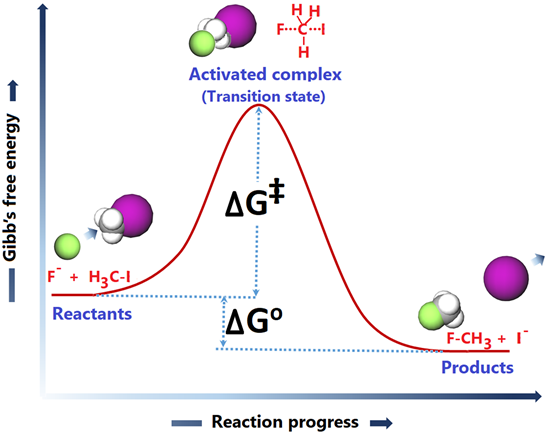
The starting point shows the energy level of reactants. Remember that every substance has internal energy which is manifested as temperature. The molecules are moving around due to the internal energy and collied with other molecules. The collision between the reactants is usually the first requirement for the reaction to proceed.
The F- ion in this example collides with the target H3C-I from the side opposite to the C-I bond. The collision at an angle other than 180o to the C-I bond is less effective or ineffective in initiating the C-I bond breaking. So, the proper orientation of the reacting molecules at the time of the collision is the second requirement.
After a properly oriented collision, the C-I bond starts breaking, the F-C bond starts making, and the energy of the system rises to a maximum level where the bond breaking and making are about halfway. The specie at the maxima of the energy curve is called activated complex or a transition state. The difference in energy between the reactants and the transition state is called activation energy (∆G‡). The reactants must have enough kinetic energy to surpass the activation energy barrier, which is the third requirement for the reaction to happen. A summary of the basic requirements of a chemical reaction to happen is the following.
- The reacting molecules collide with each other –the more frequent the collision, the faster the reaction.
- The molecules should have proper orientation at the time of the collision –this factor varies from reaction to reaction.
- The colliding molecules must have energy, in addition to the potential chemical energy, equal to or higher than the energy of activation –the lower the activation energy, the faster the reaction.
The activated complex forms if the basic requirements are fulfilled. The activated complex rolls down the hill on the energy scale and settles at the energy level of the products where the old bonds are entirely broken, and the new bonds are fully formed. The graph of the reaction progress versus the energy, as in Fig. 4.7.1 is called the reaction coordinate diagram.
Exothermic and endothermic reactions
The energy absorbed or released in the form of heat at constant pressure conditions is called enthalpy change (∆H), which is almost the same as the internal energy absorbed or released (∆G) in most of the reactions.
A chemical reaction that releases heat is exothermic. A chemical reaction that absorbs heat is an endothermic reaction.
Bond-forming is always exothermic, and bond-breaking is the opposite, i.e., endothermic, as illustrated in Fig. 4.7.2.
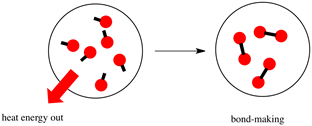

In a chemical reaction, some bonds break, and some bonds form. If the bond-breaking absorbs less heat than the heat released in the new bond making, the reaction is exothermic. The products are lower in energy than the reactants, and the ∆H is negative for exothermic reactions. The opposite is true for an endothermic reaction, i.e., the bond-breaking absorbs more heat than the bond-forming; the products are higher in energy than the reactants, and ∆H is positive, as illustrated in Fig. 4.8.3.
An example of an exothermic reaction is the combustion of methane that releases heat used for cooking.
\[\ce{CH4 + 2O2 -> CO2 + 2H2O \quad\Delta H^{o} = -891 kJ}\nonumber\]
An example of an endothermic reaction is photosynthesis.
\[\ce{6CO2 + 6H2O -> C6H12O6 + 6O2 \quad\Delta H^{o} = +2800 kJ}\nonumber\]
Note that the sign of ∆H is –ve for exothermic and +ve for an endothermic reaction. Sometimes, the energy is shown as a product for exothermic reaction, and as reactant for edothermic reaction. For example, the above two creations may be written as:
\[\ce{CH4 + 2O2 -> CO2 + 2H2O + 891 kJ}\nonumber\]
\[\ce{6CO2 + 6H2O + 2800 kJ -> C6H12O6 + 6O2}\nonumber\]

The instant hot or cold packs used in hospitals are based on the heat of dissolution of salts in water, as shown in Fig. 4.7.4. The salt and the water are in separate pockets in the pack. When the seal is ruptured, the salt dissolves in water and releases or absorbs heat. The dissolution of CaCl2 salt in water is an exothermic process that is the source of heat in a hot pack:
\[\ce{CaCl2(s) -> CaCl2(aq) \quad\Delta H^{o} = -82 kJ}\nonumber\]
The dissolution of NH4NO3 salt in water is an endothermic process that is utilized to absorb heat in a cold-pack.
\[\ce{NH4NO3(s) -> NH4NO3(aq) \quad\Delta H^{o} = -26 kJ}\nonumber\]
Factors that affect the rate of a chemical reaction
The rate of a chemical reaction, i.e., the amount of a reactant consumed or the amount of a product formed per unit time, is dependent on the energy of activation of the reaction —the higher the activation energy, the slower the rate of a reaction and vice versa. A reaction requires collision between the reactant molecules, with proper orientation, and sufficient energy to surpass the activation energy barrier.
Any factor that increases the rate of collisions, enhances the proper orientation or increases the kinetic energy of the molecules causes an increase in the rate of reaction. The factors include concentration, temperature, and catalysts.
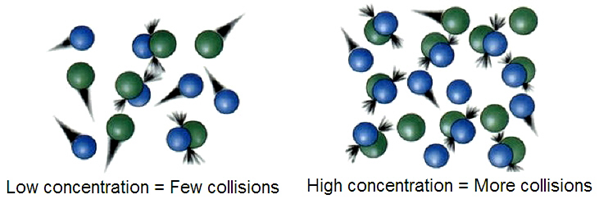
Effect of concentration of reactants
The higher the concentration, the more frequent the collisions, and the faster the reaction, as illustrated in Fig. 4.7.5.
In the breathing process, oxygen (O2) binds with hemoglobin (Hb) in the lungs.
Hb(aq) + O2(g) → HbO2(aq)
Patients having breathing problems are given breathing masks with a higher concentration of oxygen than in the atmosphere to increase the rate of oxygen binding with the hemoglobin.
Effect of temperature
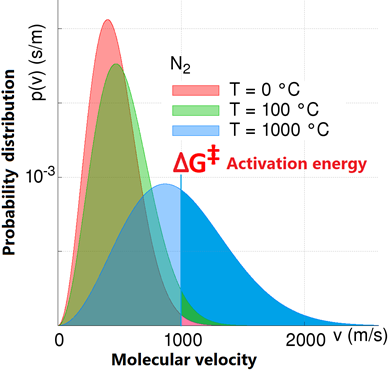
The kinetic energy of molecules at a given temperature follows Boltzman distribution, as illustrated in Fig. 4.8.6. Increased temperature increases the average kinetic energy of the molecules which increases the fraction of molecules with more than the activation energy.
An increase In temperature increases the rate of chemical reactions—generally, a 10 oC increase in temperature doubles the rate of a chemical reaction.
Some practical examples of the use of this principle are the following. Storing foods in refrigerators at lower temperatures decreases the rate of reactions resulting in longer life of the foods. Cooking foods in a pressure cooker increases the temperature resulting in faster cooking than in an open pan. In some cardiac surgeries, the body temperature is lowered to 28 oC to decrease the rate of metabolism which decreases the oxygen demand so that the heart may be stopped temporarily for the surgery.
Effect of catalysts and enzymes
The catalysts and enzymes increase the rate of reaction by decreasing the energy of activation of the reaction through an alternate route, as illustrated in Fig. 4.7.7.
The catalysts and the enzymes do not consume in the reaction -they regenerate and repeat the action. Enzymes are the catalysts in biochemical reactions. Enzymes also increase the rate of reaction by binding with the reactants and properly orienting them for the reaction.