4.5: Oxidation-reduction reactions
- Page ID
- 372944
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)What are Oxidation and Reduction?
The oxidation-reduction is defined in three ways:
- Oxidation is the loss of electrons and reduction is the gain of electrons. The word OIL RIG helps in remembering this definition.
- The addition of oxygen is oxidation, and the removal of oxygen is a reduction.
- The removal of hydrogen is oxidation, and the addition of hydrogen is a reduction.
The oxidation-reduction or in short redox reaction is one of the most common types of chemical reactions happening in and around us. For example, rusting of metals, photosynthesis, digestion of food, and combustion of fuels are redox reactions.

Oxidation-half and reduction-half reactions
The oxidation-reduction reactions happen in a pair because one thing loses electrons and is oxidized; the other thing gains the electrons and is reduced. That is why it is commonly called redox reaction, where red- represents reduction and –ox represents oxidation.
The green patina on the statue of liberty, shown in Fig. 4.5.1, is a result of the oxidation of copper:
\[\ce{2Cu(s) + O2(g) -> CuO(s)}\nonumber\]
The transfer of electrons becomes apparent when the reaction is split into the oxidation-half and reduction-half. The oxidation-half is:
\[\ce{Cu -> Cu^2+ + 2e^-}\nonumber\]
copper lost electrtons, so copper is oxides. The reduction half is:
\[\ce{O2 + 4e^- -> 2O^2-}\nonumber\]
Oxygen gained electrons, so oxygen is reduced.
- The substance that oxidizes another substance is an oxidizing agent. Oxygen is an oxidizing agent in the above reaction, as it oxidizes copper.
- The substance that reduces another substance is a reducing agent. Copper is a reducing agent in the above reaction, as it reduces oxygen.
The electrons lost in an oxidation-half must be equal to the electrons gained by the accompanying reduction-half. Multiplying the oxidation-half with 2 makes the electrons lost equal to the electrons gained in the reduction half in the above reaction. Then adding the oxidation and the reduction half gives the overall reaction:
\[\ce{2Cu -> 2Cu^2+ + \cancel{4e^-}}\nonumber\]
\[\ce{O2 + \cancel{4e^-} -> 2O^2-}\nonumber\]
\[\text {Overall reaction: } \ce{2Cu(s) + O2(g) -> CuO(s)}\nonumber\]
The chemical equations can be manipulated like algebraic equations, i.e., they can be multiplied or divided by a constant, added, and subtracted, as demonstrated in the example of the copper redox-half reactions above. Note that electrons on one side of the equation canceled the electrons on the other side of the equation during the addition operation.
A silver color zinc strip dipped in copper nitrate solution becomes coated with a layer of reddish color copper, as shown in Fig. 4.5.2. The molecular equation of the reaction, that shows formula units of compounds in the reactants and products, is:
\[\ce{Zn(s) + Cu(NO3)2(aq) -> Cu(s) + Zn(NO3)2(aq)}\nonumber\]
The complete ionic equation for the reaction is obtained by showing the dissolved ionic compounds as ions, e.g.:
\[\ce{Zn(s) + Cu^2+ (aq) + 2NO3^{-} (aq) -> Cu(s) + Zn^2+ (aq) + 2NO3^- (aq)}\nonumber\]
The oxidation-half of the reaction is:
\[\ce{Zn(s) -> Zn^2+ (aq) + 2e^-}\nonumber\]
The reduction-half of the reaction is:
\[\ce{Cu^2+ (aq) + 2e{-} -> Cu(s)}\nonumber\]
The net ionic equation that is the addition of the oxidation-half and the reduction-half is:
\[\ce{Zn(s) + Cu^2+ (aq) -> Cu(s) + Zn^2+ (aq)}\nonumber\]
Note that NO3- was on both sides of the complete ionic equation and has been canceled out in the net ionic equation:
\[\ce{Zn(s) + Cu^2+ (aq) + \cancel{2NO3^{-} (aq)} -> Cu(s) + Zn^2+ (aq) + \cancel{2NO3^- (aq)}}\nonumber\]
Ions that do not participate in the chemical reaction are called spectator ions, and they appear on both sides of the molecular equation, like NO3- in this case.
Biological oxidation and reduction reactions
In the redox reactions involving metal species, the transfer of electrons is usually evident through the oxidation-half and the reduction-half reactions, as in the above examples. In the organic and biochemical redox reactions, the transfer of electrons is usually not so obvious, but the transfer of hydrogen or oxygen is usually apparent. For example, the metabolism of methanol \(\ce{H3C-OH}\) starts with oxidation through the loss of hydrogen:
\[\ce{H3C-OH -> H2C=O + 2H}\nonumber\]
Metanol is oxidized to formaldehyde \(\ce{H2C=O}\). Formaldehyde oxidizes further by gaining oxygen:
\[\ce{H2C=O -> HCOOH}\nonumber\]
Finally, the formic acid (HCOOH) is oxidized by gaining oxygen and forming carbon dioxide and water:
\[\ce{2HCOOH -> 2O=C=O + 2H2O}\nonumber\]
The increase in the C-O bond from a single bond in methanol to two double bonds (four C-O bonds) in carbon dioxide is a clear indication of the oxidation of the carbon.
Removal of hydrogen is also oxidation, e.g., hydroxymalonate is oxidized to oxomalonate by an enzyme hydroxymalonate dehydrogenase:
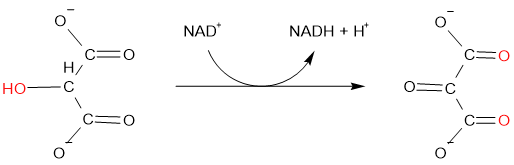
The reverse of these, i.e., removal of oxygen and addition of hydrogen is reduction.


