Chlorides of Period 3 Elements
- Page ID
- 3666
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)This page discusses the structures of the chlorides of the Period 3 elements (sodium to sulfur), their physical properties and their reactions with water. Chlorine and argon are omitted—chlorine because it is meaningless to talk about "chlorine chloride", and argon because it is inert and does not form a chloride.
A quick summary of the trends
The chlorides of interest are given in the table below:
| NaCl | MgCl2 | AlCl3 | SiCl4 | PCl5 | S2Cl2 |
| PCl3 |
Sulfur forms three chlorides, but S2Cl2 is most common. Aluminum chloride also exists under some conditions as a dimer, Al2Cl6.
- The structures: Sodium chloride and magnesium chloride are ionic and consist of large ionic lattices at room temperature. Aluminum chloride and phosphorus(V) chloride are more complicated. They change their structures from ionic to covalent as their solids transition to liquids or vapors. This is discussed in greater detail below. The other chlorides are simple covalent molecules.
- Melting and boiling points: Sodium and magnesium chlorides are solids with high melting and boiling points because of the large amount of heat which is needed to break the strong ionic attractions.
- The rest are liquids or low melting point solids. Leaving aside the aluminum chloride and phosphorus(V) chloride cases where the situation is quite complicated, the attractions in the others will be much weaker intermolecular forces such as van der Waals dispersion forces. These vary depending on the size and shape of the molecule, but will always be far weaker than ionic bonds.
- Electrical conductivity: Sodium and magnesium chlorides are ionic and so will undergo electrolysis when they are molten. Electricity is carried by the movement of the ions and their discharge at the electrodes (not electrons). In the aluminum chloride and phosphorus(V) chloride cases, the solid does not conduct electricity because the ions aren't free to move. In the liquid (where it exists - both of these sublime at ordinary pressures), they have converted into a covalent form, and so don't conduct either. The rest of the chlorides do not conduct electricity either solid or molten because they don't have any ions or any mobile electrons.
- Reactions with water: As an approximation, the simple ionic chlorides (sodium and magnesium chloride) just dissolve in water. Although other chlorides all react with water in a variety of ways described below for each individual chloride. The reaction with water is known as hydrolysis.
Sodium chloride (NaCl)
Sodium chloride is an ionic compound consisting of a giant array of sodium and chloride ions. A small representative portion of a sodium chloride lattice looks like this:
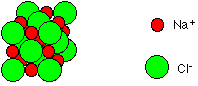
This is normally drawn in an exploded form as:
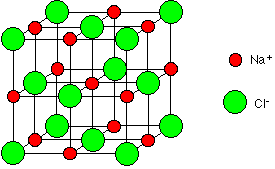
The strong attractions between the positive and negative ions require a large amount of heat energy to break, so sodium chloride has high melting and boiling points. The compound does not conduct electricity in the solid state because it has no mobile electrons, and the ions are constrained by the crystal lattice. However, when it melts it undergoes electrolysis. Sodium chloride dissolves in water to give a neutral solution.
Magnesium chloride (MgCl2)
Like sodium chloride, magnesium chloride also forms an ionic solid, but with a more complicated crystal structure of the ions to accommodate twice as many chloride ions as magnesium ions. As with sodium chloride, large amounts of heat energy are needed to overcome the attractions between the ions (because of the high lattice enthalpy of the compound), so the melting and boiling points are also high. Solid magnesium chloride is a non-conductor of electricity because the ions are constrained. However, upon melting, the compound undergoes electrolysis.
Magnesium chloride dissolves in water to give a slightly acidic solution (with a pH of approximately 6). When magnesium ions are solvated from the solid lattice, there is enough attraction between the 2+ ions and the water molecules to form coordinate (dative covalent) bonds between the magnesium ions and lone pairs on surrounding water molecules. Hexaaquamagnesium complex ions are formed, [Mg(H2O)6]2+, as follows:
\[ MgCl_{2 (s)} + 6H_2O \rightarrow [Mg(H_2O)_6]^{2+}_{(aq)} + 2Cl^-_{(aq)} \nonumber \]
Many complex ions are acidic, the degree of acidity depending on the attraction between the electrons in the water molecules and the metal at the center of the ion. The hydrogen atoms carry less electron density in this state, and are thus more easily removed by a base. For magnesium, the amount of distortion is quite small, and only a small proportion of the hydrogen atoms are removed, in this case by water molecules in the solution:
\[ [Mg(H_2O)_6]^{2+} + H_2O_{(l)} \rightleftharpoons [Mg(H_2O)_5(OH)^{2+}]^+ +H_3O^+_{(aq)} \nonumber \]
The hydronium ions make the solution acidic. Few are formed (the equilibrium lies well to the left) because the solution is only weakly acidic. The previous equation can be simplified as follows:
\[ [Mg(H_2O)^6]^{2+}_{(aq)} \rightleftharpoons [Mg(H_2O)_5(OH)]^+_{(aq)} + H^+_{(aq)} \nonumber \]
It is essential to include the state symbols if the equation is written this way.
Aluminum chloride (AlCl3)
Electronegativity increases across the period; aluminum and chlorine do not differ enough in electronegativity to form a simple ionic bond. The structure of aluminum chloride changes with temperature. At room temperature, the aluminum is 6-coordinated (i.e. each aluminum is surrounded by 6 chlorine atoms). The structure is an ionic lattice, but it has a lot of covalent character.
At atmospheric pressure, aluminum chloride sublimes at about 180°C. If the pressure is increased to just over 2 atmospheres, it melts instead at a temperature of 192°C.
Both of these temperatures are far below the expected range for an ionic compound. They suggest comparatively weak attractions between molecules instead of strong attractions between ions. This is because the coordination of the aluminum changes at these temperatures. It becomes 4-coordinated—each aluminum is surrounded by 4 chlorine atoms rather than 6. The original lattice converts into an Al2Cl6 arrangement of molecules. The structure is shown below:
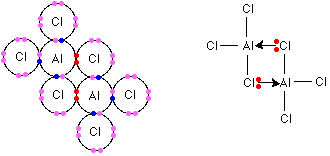
In the conversion, all ionic character is lost, causing the aluminum chloride to vaporize or melt (depending on the pressure). These dimers and simple AlCl3 molecules exist in equilibrium. As the temperature increases further, the position of equilibrium shifts more and more to the right of the following system:
\[ Al_2Cl_6 \rightleftharpoons 2AlCl_3 \nonumber \]
Summary of AlCl3
- At room temperature, solid aluminum chloride has an ionic lattice with significant of covalent character.
- At temperatures around 180 - 190°C (depending on the pressure), aluminum chloride coverts to its molecular form, Al2Cl6. This causes it to melt or vaporize due to comparatively weak intermolecular attractions.
- As the temperature increases further, more AlCl3 molecules are formed.
Solid aluminum chloride does not conduct electricity at room temperature because the ions are not free to move. Molten aluminum chloride (only possible at increased pressures) is also nonconductive, because it has lost its ionic character.
Aluminum chloride reacts dramatically with water. A drop of water placed onto solid aluminum chloride produces steamy clouds of hydrogen chloride gas. Solid aluminum chloride in an excess of water still splutters, but instead an acidic solution is formed. A solution of aluminum chloride of ordinary concentrations (around 1 mol dm-3, for example) has a pH around 2-3. More concentrated solutions have a lower pH.
The aluminum chloride reacts with the water rather than simply dissolving in it. In the first instance, hexaaquaaluminum complex ions and chloride ions are formed:
\[ AlCl_3 (s) + 6H_2O (l) \rightarrow [Al(H_2O)_6]^{3+} (aq) + 3Cl^- (aq) \nonumber \]
This is very similar to the magnesium chloride equation given above—the only difference is the charge on the ion. The greater charge attracts electrons in the water molecules quite strongly toward the aluminum, making the hydrogen atoms more positive and therefore easier to remove from the ion. Hence, this ion is much more acidic than in the corresponding magnesium case.
The acid-base equilibria for this reaction lie further to the right than those for magnesium, and so the solution formed is more acidic—more hydronium ions are formed, as shown:
\[ [Al(H_2O)_6]^{3+} + H_2O \rightleftharpoons [Al(H_2O)_5(OH)]^{2+} + H_3O^+ \nonumber \]
or, more simply:
\[ [Al(H_2O)_6]^{3+} (aq) \rightleftharpoons [Al(H_2O)_5(OH)]^{2+} (aq) + H^+ \nonumber \]
If there is little water present, hydrogen chloride gas is produced. Because of the heat produced in the reaction and the concentration of the solution formed, hydrogen ions and chloride ions in the mixture combine together as hydrogen chloride (\(HCl\)) molecules and are given off as a gas. In a large excess of water, the temperature is never high enough for this to happen; the ions remain solvated.
Silicon tetrachloride (SiCl4)
Silicon tetrachloride is a simple no-messing-about covalent chloride. There isn't enough electronegativity difference between the silicon and the chlorine for the two to form ionic bonds. Silicon tetrachloride is a colorless liquid at room temperature which fumes in moist air. The only attractions between the molecules are van der Waals dispersion forces. It doesn't conduct electricity because of the lack of ions or mobile electrons.
It fumes in moist air because it reacts with water in the air to produce hydrogen chloride. If you add water to silicon tetrachloride, there is a violent reaction to produce silicon dioxide and fumes of hydrogen chloride. In a large excess of water, the hydrogen chloride will, of course, dissolve to give a strongly acidic solution containing hydrochloric acid.
\[ SiCl_4 + 2H_2O \rightarrow SiO_2 + 4HCl \nonumber \]
The phosphorus chlorides
There are two phosphorus chlorides: phosphorus(III) chloride, PCl3, and phosphorus(V) chloride, PCl5.
Phosphorus(III) chloride (PCl3)
This simple covalent chloride exists as a fuming liquid at room temperature because there are only van der Waals dispersion forces and dipole-dipole attractions between the molecules. The liquid does not conduct electricity because of the lack of ions or mobile electrons.
Phosphorus(III) chloride reacts violently with water to generate phosphorous acid, H3PO3, and hydrogen chloride fumes (or a solution containing hydrochloric acid in excess of water):
\[ PCl_3 + 3H_2O \rightarrow H_3PO_3 + 3HCl \nonumber \]
Phosphorus(V) chloride (PCl5)
Phosphorus(V) chloride is structurally more complicated than phosphorus(III) chloride. At room temperature, it forms a white solid which sublimes at 163°C. Increasing the temperature beyond its sublimation point dissociates (divides reversibly) more the phosphorus(V) chloride into phosphorus(III) chloride and chlorine:
\[ PCl_5 \rightleftharpoons PCl_3 + Cl_2 \nonumber \]
Phosphorus(V) chloride is an ionic solid. The formation of the ions involves two molecules of PCl5. A chloride ion transfers from one of the original molecules to the other, leaving a positive ion, [PCl4]+, and a negative ion, [PCl6]-.
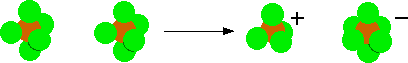
At 163°C, the phosphorus(V) chloride converts to a molecular form containing PCl5 molecules. Because only van der Waals dispersion forces exist between these molecules, the species vaporizes. Solid phosphorus(V) chloride does not conduct electricity.
Phosphorus(V) chloride reacts violently with water, producing hydrogen chloride fumes. As with the other covalent chlorides, if there is enough water present, these dissolve to give a hydrochloric acid solution.
The reaction happens in two stages. The first takes place in cold water; phosphorus oxychloride, POCl3, is produced along with HCl:
\[ PCl_5 + 4H_2O \rightarrow POCl_3 + 2HCl \nonumber \]
As the solution is brought to a boil, the phosphorus(V) chloride reacts further to give phosphoric(V) acid and more HCl. Phosphoric(V) acid is also known as phosphoric acid or as orthophosphoric acid:
\[ POCl_3 + 3H_2O \rightarrow H_3PO_4 + 3HCl \nonumber \]
Combining these equations gives the overall reaction in boiling water:
\[ PCl_5 + 4H_2O \rightarrow H_3PO_4 + 5HCl \nonumber \]
Disulfur Dichloride (S2Cl2)
Disulfur dichloride is one of three sulfur chlorides and is the species formed when chlorine reacts with hot sulfur. Disulfur dichloride is an orange, unpleasant-smelling covalent liquid. Its rather unusual structure is given below:
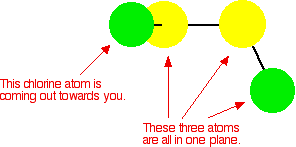
The molecule's conformation indicates its possible intermolecular interactions:
- There is no plane of symmetry in the molecule; therefore, it has an overall permanent dipole.
- In liquid state, the molecule experiences van der Waals dispersion forces and dipole-dipole attractions.
- There are no ions in disulfur dichloride and no mobile electrons, making it nonconductive.
Disulfur dichloride reacts slowly with water to produce a complex mixture of hydrochloric acid, sulfur, hydrogen sulfide and various sulfur-containing acids and anions.
Contributors and Attributions
Jim Clark (Chemguide.co.uk)


