Acid-base Behavior of the Oxides
- Page ID
- 3665
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)This page discusses the reactions of the oxides of Period 3 elements (sodium to chlorine) with water, and with acids or bases where relevant (as before, argon is omitted because it does not form an oxide).
A quick summary of the trend
The oxides: The oxides of interest are given below:
| Na2O | MgO | Al2O3 | SiO2 | P4O10 | SO3 | Cl2O7 |
| P4O6 | SO2 | Cl2O |
The trend in acid-base behavior can be summarized as follows:
Acidity increases from left to right, ranging from strongly basic oxides on the left to strongly acidic ones on the right, with an amphoteric oxide (aluminum oxide) in the middle. An amphoteric oxide is one which shows both acidic and basic properties.
This trend applies only to the highest oxides of the individual elements (see the top row of the table), in the highest oxidation states for those elements. The pattern is less clear for other oxides. Non-metal oxide acidity is defined in terms of the acidic solutions formed in reactions with water—for example, sulfur trioxide reacts with water to forms sulfuric acid. They will all, however, react with bases such as sodium hydroxide to form salts such as sodium sulfate as explored in detail below.
Sodium Oxide
Sodium oxide is a simple strongly basic oxide. It is basic because it contains the oxide ion, O2-, which is a very strong base with a high tendency to combine with hydrogen ions.
Reaction with water: Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution. A concentrated solution of sodium oxide in water will have pH 14.
\[ Na_2O + H_2O \rightarrow 2NaOH \nonumber \]
Reaction with acids: As a strong base, sodium oxide also reacts with acids. For example, it reacts with dilute hydrochloric acid to produce sodium chloride solution.
\[Na_2O + 2HCl \rightarrow 2NaCl + H_2O \nonumber \]
Magnesium oxide
Magnesium oxide is another simple basic oxide, which also contains oxide ions. However, it is not as strongly basic as sodium oxide because the oxide ions are not as weakly-bound. In the sodium oxide, the solid is held together by attractions between 1+ and 2- ions. In magnesium oxide, the attractions are between 2+ and 2- ions. Because of the higher charge on the metal, more energy is required to break this association. Even considering other factors (such as the energy released from ion-dipole interactions between the cations and water), the net effect is that reactions involving magnesium oxide will always be less exothermic than those of sodium oxide.
Reaction with water: At first glance, magnesium oxide powder does not appear to react with water. However, the pH of the resulting solution is about 9, indicating that hydroxide ions have been produced. In fact, some magnesium hydroxide is formed in the reaction, but as the species is almost insoluble, few hydroxide ions actually dissolve. The reaction is shown below:
\[MgO + H_2O \rightarrow Mg(OH)_2 \nonumber \]
Reaction with acids: Magnesium oxide reacts with acids as predicted for a simple metal oxide. For example, it reacts with warm dilute hydrochloric acid to give magnesium chloride solution.
\[MgO + 2HCl \rightarrow MgCl_2+H_2O \nonumber \]
Aluminum Oxide
Describing the properties of aluminum oxide can be confusing because it exists in a number of different forms. One of those forms is very unreactive (known chemically as alpha-Al2O3) and is produced at high temperatures. The following reactions concern the more reactive forms of the molecule. Aluminium oxide is amphoteric. It has reactions as both a base and an acid.
Reaction with water: Aluminum oxide is insoluble in water and does not react like sodium oxide and magnesium oxide. The oxide ions are held too strongly in the solid lattice to react with the water.
Reaction with acids: Aluminum oxide contains oxide ions, and thus reacts with acids in the same way sodium or magnesium oxides do. Aluminum oxide reacts with hot dilute hydrochloric acid to give aluminum chloride solution.
\[Al_2O_3 + 6HCl \rightarrow 2AlCl_3 + 3H_2O \nonumber \]
This reaction and others display the amphoteric nature of aluminum oxide.
Reaction with bases: Aluminum oxide also displays acidic properties, as shown in its reactions with bases such as sodium hydroxide. Various aluminates (compounds in which the aluminum is a component in a negative ion) exist, which is possible because aluminum can form covalent bonds with oxygen. This is possible because the electronegativity difference between aluminum and oxygen is small, unlike the difference between sodium and oxygen, for example (electronegativity increases across a period)
Aluminum oxide reacts with hot, concentrated sodium hydroxide solution to produce a colorless solution of sodium tetrahydroxoaluminate:
\[Al_2O_3 + 2NaOH +3H_2O \rightarrow 2NaAl(OH)_4 \nonumber \]
Silicon dioxide (silicon(IV) oxide)
Silicon is too similar in electronegativity to oxygen to form ionic bonds. Therefore, because silicon dioxide does not contain oxide ions, it has no basic properties. In fact, it is very weakly acidic, reacting with strong bases.
Reaction with water: Silicon dioxide does not react with water, due to the thermodynamic difficulty of breaking up its network covalent structure.
Reaction with bases: Silicon dioxide reacts with hot, concentrated sodium hydroxide solution, forming a colorless solution of sodium silicate:
\[SiO_2 + 2NaOH \rightarrow Na_2SiO_3 + H2O \nonumber \]
In another example of acidic silicon dioxide reacting with a base, the Blast Furnace extraction of iron, calcium oxide from limestone reacts with silicon dioxide to produce a liquid slag, calcium silicate:
\[SiO_2 + CaO \rightarrow CaSiO_3 \nonumber \]
Phosphorus Oxides
Two phosphorus oxides, phosphorus(III) oxide, P4O6, and phosphorus(V) oxide, P4O10, are considered here.
Phosphorus(III) oxide: Phosphorus(III) oxide reacts with cold water to produce a solution of the weak acid, H3PO3—known as phosphorous acid, orthophosphorous acid or phosphonic acid:
\[P_4O_6 + 6H_2O \rightarrow 4H_3PO_3 \nonumber \]
The fully-protonated acid structure is shown below:
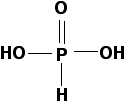
The protons remain associated until water is added; even then, because phosphorous acid is a weak acid, few acid molecules are deprotonated. Phosphorous acid has a pKa of 2.00, which is more acidic than common organic acids like ethanoic acid (pKa = 4.76).
Phosphorus(III) oxide is unlikely to be reacted directly with a base. In phosphorous acid, the two hydrogen atoms in the -OH groups are acidic, but the third hydrogen atom is not. Therefore, there are two possible reactions with a base like sodium hydroxide, depending on the amount of base added:
\[ NaOH + H_3PO_3 \rightarrow NaH_2PO_3 + H_2O \nonumber \]
\[ 2NaOH + H_3PO_3 \rightarrow Na_2HPO_3 + 2H_2O \nonumber \]
In the first reaction, only one of the protons reacts with the hydroxide ions from the base. In the second case (using twice as much sodium hydroxide), both protons react.
If instead phosphorus(III) oxide is reacted directly with sodium hydroxide solution, the same salts are possible:
\[4NaOH + P_4O_6 + 2H_2O \rightarrow 4NaH_2PO_3 \nonumber \]
\[9NaOH + P_4O_6 \rightarrow 4Na_2HPO_3 + 2H_2O \nonumber \]
Phosphorus(V) oxide: Phosphorus(V) oxide reacts violently with water to give a solution containing a mixture of acids, the nature of which depends on the reaction conditions. Only one acid is commonly considered, phosphoric(V) acid, H3PO4 (also known as phosphoric acid or as orthophosphoric acid).
\[P_4O_{10} + 6H_2O \rightarrow 4H_3PO_4 \nonumber \]
This time the fully protonated acid has the following structure:
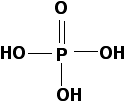
Phosphoric(V) acid is another weak acid with a pKa of 2.15, marginally weaker than phosphorous acid. Solutions of each of these acids with concentrations around 1 mol dm-3 have a pH of about 1.
Phosphoric (V) oxide is also unlikely to be reacted directly with a base, but the hypothetical reactions are considered. In its acid form, molecule has three acidic -OH groups, which can cause a three-stage reaction with sodium hydroxide:
\[ NaOH + H_3PO_4 \rightarrow NaH_2PO_4 + H_2O \nonumber \]
\[ 2NaOH + H_3PO_4 \rightarrow Na_2HPO_4 + 2H_2O \nonumber \]
\[ 3NaOH + H_3PO_4 \rightarrow Na_3PO_4 + 3H_2O \nonumber \]
Similar to phosphorus (III) oxide, if phosphorus(V) oxide reacts directly with sodium hydroxide solution, the same possible salt as in the third step (and only this salt) is formed:
\[12NaOH + P_4O_{10} \rightarrow 4Na_3PO_4 + 6H_2O \nonumber \]
Sulfur Oxides
Two oxides are considered: sulfur dioxide, SO2, and sulfur trioxide, SO3.
Sulfur dioxide: Sulfur dioxide is fairly soluble in water, reacting to give a solution of sulfurous acid (also known as sulfuric(IV) acid), H2SO3, as shown in the reaction below. This species only exists in solution, and any attempt to isolate it gives off sulfur dioxide.
\[ SO_2 + H_2O \rightarrow H_2SO_3 \nonumber \]
The protonated acid has the following structure:
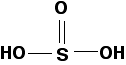
Sulfurous acid is also a relatively weak acid, with a pKa of around 1.8, but slightly stronger than the two phosphorus-containing acids above. A reasonably concentrated solution of sulfurous acid has a pH of about 1.
Sulfur dioxide also reacts directly with bases such as sodium hydroxide solution. Bubbling sulfur dioxide through sodium hydroxide solution first forms sodium sulfite solution, followed by sodium hydrogen sulfite solution if the sulfur dioxide is in excess.
\[ SO_2 + 2NaOH \rightarrow Na_2SO_3 + H_2O \nonumber \]
\[Na_2SO_3 + H_2O \rightarrow 2NaHSO_3 \nonumber \]
Another important reaction of sulfur dioxide is with the base calcium oxide to form calcium sulfite (also known as calcium sulfate(IV)). This is of the important methods of removing sulfur dioxide from flue gases in power stations.
\[CaO + SO_2 \rightarrow CaSO_3 \nonumber \]
Sulfur trioxide: Sulfur trioxide reacts violently with water to produce a fog of concentrated sulfuric acid droplets.
\[ SO_3 + H_2O \rightarrow H_2SO_4 \nonumber \]
Pure, fully-protonated sulfuric acid has the structure:
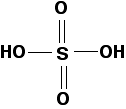
Sulfuric acid is a strong acid, and solutions will typically have a pH around 0. The acid reacts with water to give a hydronium ion (a hydrogen ion in solution) and a hydrogen sulfate ion. This reaction runs essentially to completion:
\[ H_2SO_4 (aq) + H_2O (l) \rightarrow H_3P^+ + HSO_4^- (aq) \nonumber \]
The second proton is more difficult to remove. In fact, the hydrogen sulfate ion is a relatively weak acid, similar in strength to the acids discussed above. This reaction is more appropriately described as an equilibrium:
\[ HSO_4^- (aq) + H_2O \rightleftharpoons H_3O^+ (aq) + SO_4^{2-} (aq) \nonumber \]
It is useful if you understand the reason that sulfuric acid is a stronger acid than sulfurous acid. You can apply the same reasoning to other acids that you find on this page as well.
Sulfuric acid is stronger than sulfurous acid because when a hydrogen ion is lost from one of the -OH groups on sulfuric acid, the negative charge left on the oxygen is spread out (delocalized) over the ion by interacting with the doubly-bonded oxygen atoms. It follows that more double bonded oxygen atoms in the ion make more delocalization possible; more delocalization leads to greater stability, making the ion less likely to recombine with a hydrogen ion and revert to the non-ionized acid.
Sulfurous acid only has one double bonded oxygen, whereas sulfuric acid has two; the extra double bond provides much more effective delocalization, a much more stable ion, and a stronger acid. Sulfuric acid displays all the reactions characteristic of a strong acid. For example, a reaction with sodium hydroxide forms sodium sulfate; in this reaction, both of the acidic protons react with hydroxide ions as shown:
\[2NaOH +H_2SO_4 \rightarrow Na_2SO_4 + 2H_2O \nonumber \]
In principle, sodium hydrogen sulfate can be formed by using half as much sodium hydroxide; in this case, only one of the acidic hydrogen atoms is removed.
Sulfur trioxide itself also reacts directly with bases such as calcium oxide, forming calcium sulfate:
\[ CaO + SO_3 \rightarrow CaSO_4 \nonumber \]
This reaction is similar to the reaction with sulfur dioxide discussed above.
Chlorine Oxides
Chlorine forms several oxides, but only two (chlorine(VII) oxide, Cl2O7, and chlorine(I)oxide, Cl2O) are considered here. Chlorine(VII) oxide is also known as dichlorine heptoxide, and chlorine(I) oxide as dichlorine monoxide.
Chlorine(VII) oxide: Chlorine(VII) oxide is the highest oxide of chlorine—the chlorine atom is in its maximum oxidation state of +7. It continues the trend of the highest oxides of the Period 3 elements towards being stronger acids. Chlorine(VII) oxide reacts with water to give the very strong acid, chloric(VII) acid, also known as perchloric acid.
\[ Cl_2O_7 + H_2O \rightarrow 2HClO_4 \nonumber \]
As in sulfuric acid, the pH of typical solutions of perchloric acid are around 0. Neutral chloric(VII) acid has the following structure:
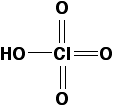
When the chlorate(VII) ion (perchlorate ion) forms by loss of a proton (in a reaction with water, for example), the charge is delocalized over every oxygen atom in the ion. That makes the ion very stable, making chloric(VII) acid very strong.
Chloric(VII) acid reacts with sodium hydroxide solution to form a solution of sodium chlorate(VII):
\[ NaOH + HClO_4 \rightarrow NaClO_4 + H2O \nonumber \]
Chlorine(VII) oxide itself also reacts directly with sodium hydroxide solution to give the same product:
\[ 2NaOH + Cl_2O_7 \rightarrow 2NaClO_4 + H_2O \nonumber \]
Chlorine(I) oxide: Chlorine(I) oxide is far less acidic than chlorine(VII) oxide. It reacts with water to some extent to give chloric(I) acid, \(HOCl^-\) also known as hypochlorous acid.
\[ Cl_2O + H_2O \rightleftharpoons 2HOCl \nonumber \]
The structure of chloric(I) acid is exactly as shown by its formula, HOCl. It has no doubly-bonded oxygens, and no way of delocalizing the charge over the negative ion formed by loss of the hydrogen. Therefore, the negative ion formed not very stable, and readily reclaims its proton to revert to the acid. Chloric(I) acid is very weak (pKa = 7.43) and reacts with sodium hydroxide solution to give a solution of sodium chlorate(I) (sodium hypochlorite):
\[ NaOH + HOCl \rightarrow NaOCl + H_2O \nonumber \]
Chlorine(I) oxide also reacts directly with sodium hydroxide to give the same product:
\[2NaOH + Cl_2O \rightarrow 2NaOCl + H_2O \nonumber \]
Contributor
Jim Clark (Chemguide.co.uk)


